Translate this page into:
Bronchial Artery Aneurysm due to Pulmonary Tuberculosis: Detection with Multidetector Computed Tomographic Angiography
Address for correspondence: Dr. Zafar Neyaz, Department of Radiodiagnosis, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Rae Bareilly Road, Lucknow, Uttar Pradesh – 226 014, India. E-mail: zafarneyaz@hotmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A case of bronchial artery aneurysm due to pulmonary tuberculosis is reported. The patient presented with massive hemoptysis and the diagnosis was made using multidetector computed tomographic (MDCT) angiography. Selective bronchial arteriogram confirmed the MDCT findings and bronchial artery embolization was successfully performed with cessation of hemoptysis. Our article emphasizes the value of MDCT angiography in the diagnosis and management of such cases.
Keywords
Bronchial artery aneurysm
hemoptysis
multidetector computed tomographic angiography
therapeutic embolization
tuberculosis
INTRODUCTION

While the prevalence of pulmonary tuberculosis has declined in developed nations, it still remains a widely prevalent disease in South East Asia and Africa.[1] Hemoptysis is a serious complication of pulmonary tuberculosis. Angiographic findings in tuberculosis patients presenting with massive hemoptysis include hypervascularity, hypertrophy of systemic arteries, aneurysm, systemic to pulmonary anastomosis, and rarely, contrast extravasation.[2] Bronchial arteries are the source of hemorrhage in majority of these cases with non-bronchial systemic or pulmonary arteries being less common as the source.
Multidetector computed tomographic (MDCT) angiography is proving to be a robust tool in identifying the cause of hemoptysis as well as in viewing enlarged bronchial or non-bronchial systemic arteries.[34] Bronchial artery aneurysms are nicely depicted with MDCT.[35–9] We describe a case of bronchial artery aneurysm in a patient with pulmonary tuberculosis presenting with massive hemoptysis and emphasize the role of MDCT angiography in diagnosis.
CASE REPORT
A 56-year-old male presented with massive hemoptysis for 2 days. He had been experiencing low grade fever and recurrent episodes of streaky hemoptysis for 5 months. He was managed conservatively at a peripheral centre and was referred to our institute with ongoing episodes of hemoptysis amounting to 15–20 ml per episode. He had been previously treated for pulmonary tuberculosis about 18 years earlier. The hemogram, coagulogram, and routine blood biochemistry were within normal limits: hemoglobin of 8.2 g/dl, total leukocyte count of 7900/mm3 (70% neutrophils, 26% lymphocytes, 2% monocytes, 2% eosinophils) and erythrocyte sedimentation rate (ESR) of 65 mm in the first hour. Platelet count and coagulation profile were normal.
A chest radiograph showed fibrotic opacities and few thin-walled cavities in the left upper zone and an ill-defined nodular opacity in the right middle zone. MDCT angiography was done on a 64-channel multidetector scanner (Brilliance CT, Philips Medical systems, Cleveland, OH, USA) at our institution. The imaging parameters were: detector collimation, 64 × 0.625 mm; pitch, 1.108; gantry rotation time, 0.75 second; reconstructed section thickness, 0.9 mm; reconstruction interval, 0.45 mm; field of view, 350 mm; filter, sharp C; window center, 50; window width, 350; tube voltage, 120 kV; and planned tube current-time product, 250 mAs. High resolution lung window images were obtained with the following parameters: reconstruction section thickness, 0.8 mm; reconstruction interval, 0.4 mm; filter Y sharp; window center, -500, and window width, 1500. Through an intravenous antecubital catheter, 70 ml of nonionic contrast (Iomeron, 400 mg of iodine per ml; Bracco Pharmaceuticals, Milan, Italy) was injected by a power injector (Stellant; Medrad, Indianola, PA) with a flow rate of 4.5 ml/sec, followed by 40 ml saline chase at the rate of 4.0 ml/sec. The region of interest was placed in the descending thoracic aorta, and scanning was automatically initiated once a selected threshold (150 HU) was reached using a bolus tracking method.
MDCT revealed a necrotic nodular lesion and associated fibrotic components in the right middle lobe with areas of ground glass appearance and scattered tiny nodules in adjacent lung parenchyma. Small pocket of air was also present inside the necrotic lesion. MDCT angiography images showed multiple hypertrophied arteries inside the lesion with a small aneurysm (3 × 5 mm) [Figure 1a]. The right bronchial artery was mildly enlarged and could be traced to the parenchymal abnormality in the right middle lobe [Figure 1b]. MDCT also revealed fibrotic opacities in the left upper lobe with two small, thin-walled cavities and bronchiectatic changes in the left upper lobe [Figure 1c]. Based on clinical history and radiological findings, a diagnosis of reactivate tuberculosis with aneurysm formation was made.
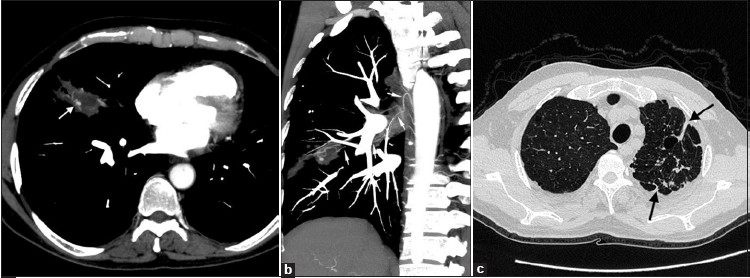
- Multidetector CT angiogram. (a) Axial maximum-intensity-projection image shows a necrotic nodular lesion with fibrotic components in the right middle lobe. Tortuous hypertrophied arteries and a tiny aneurysm are noted inside the lesion (arrow). (b) Coronal oblique maximum-intensity-projection image shows mildly enlarged right bronchial artery arising from right intercostobronchial trunk and coursing to the area of parenchymal abnormality (thin arrows).(c) MDCT showing fibrotic opacities in the left upper lobe with two small, thin-walled cavities and bronchiectatic changes
The right bronchial artery was catheterized with a 5-F renal curve catheter. Selective arteriography confirmed the diagnosis of aneurysm of the right bronchial artery [Figure 2a]. Then, a microcatheter (Asahi Intec, Moriyama-ku, Nagoya-shi, Aichi 463-0024,Japan ) was advanced coaxially into the right bronchial artery and therapeutic embolization was performed with 300–500 μm polyvinyl alcohol (PVA) particles (Contour, Boston Scientific, Natick, MA 01760-1537, USA). Post-embolization angiogram showed occlusion of feeding vessels and aneurysm [Figure 2b]. Patient was put on antimycobacterial drugs and discharged 2 days later. On follow-up after 2 months, the patient had no recurrence of hemoptysis.
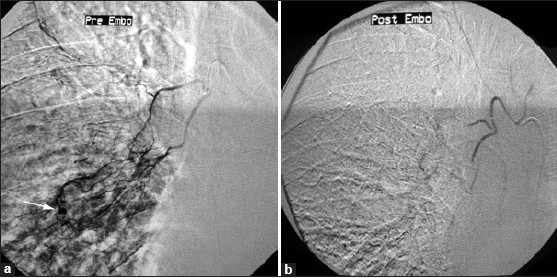
- Superselective embolization with a microcatheter. (a) Selective arteriogram of the right bronchial artery shows hypervascularity in right middle zone with enlarged arteries and a small aneurysm (arrow). (b) After embolization with PVA particles, the hypervascular area and the aneurysm are not visualized.
DISCUSSION
Chronic inflammation associated with tubercular lesions leads to neovascularization and increased collateral supply from nearby systemic arteries.[3] Granulocyte-Colony Stimulating Factor, Granulocyte Macrophage Colony Stimulating Factor, Basic Fibroblast Growth Factor, and Vascular Endothelial Growth Factor (most potent) have been implicated as direct mediators of angiogenesis and neovascularization. Prostaglandins E1 and E2 (PGE1, PGE2), Tumour Necrosis Factor α (TNF α), Interleukins 1, 6, and 8 (IL-1, IL-6, IL-8) and Nitric Oxide are inflammatory mediators that have indirect angiogenic activity.[4] These newly formed collateral vessels have weak arterial wall and are prone to rupture. Tubercular cavities and tubercular bronchiectasis are often the source of bleeding.[2–10] Hypervascularity and hypertrophied bronchial arteries are the common findings on angiograms. Bronchial artery aneurysms have been reported in about 7% of patients of pulmonary tuberculosis presenting with massive hemoptysis.[2] The term pseudoaneurysm is often used interchangeably with the aneurysm.[11] Bronchial artery aneurysm has also been associated with congenital causes such as pulmonary sequestration or pulmonary agenesis, or acquired causes like atherosclerosis, inflammatory lung disease, bronchiectasis, sarcoidosis, Osler-Weber-Rendu disease, and trauma.[6–10] Bronchial artery aneurysms may arise either within the mediastinum or from the intrapulmonary portion of the artery.[11] Rupture of bronchial artery aneurysms located in mediastinum may cause hemothorax and mediastinal hemorrhage whereas rupture of the intrapulmonary aneurysm can give rise to massive hemoptysis. Rarely, an aneurysm of the pulmonary artery (Rasmussen aneurysm) is also caused by erosion from adjacent tubercular cavities.[12]
MDCT angiography is a non-invasive investigation that enables detailed evaluation of the lung parenchyma, airway and mediastinum along with the thoracic vasculature in a single study.[3] CT scan helps in localizing the site of hemorrhage in 63–100% of patients with hemoptysis, a rate that is higher than that for fibro-optic bronchoscopy (FOB).[11] It shows distal airways beyond the reach of the FOB and the lung parenchyma surrounding these airways.[13] However, FOB is more useful than CT in evaluating endobronchial lesions and early mucosal abnormalities. Another advantage of FOB is that it can be used to localize the site of hemoptysis in patients whose condition is not stable enough to allow them to leave the intensive care unit to undergo CT. FOB can be used to give local therapy for control of hemorrhage and to provide sample for tissue diagnosis and microbiology work up. Thus, FOB remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis. Although in a case of massive hemoptysis, the patient can be directly taken up for combined diagnostic angiography and bronchial artery embolization without CT scan and bronchoscopy, this approach has some disadvantages.[13] First, conventional angiography is not as good as CT scan for localization and characterization of the source of hemorrhage. Second, performing CT first can rule out situations in which surgery would be preferred over bronchial artery embolization. Compared with conventional angiography, MDCT angiography is a minimally invasive, quick, and relatively low cost procedure. In emergency situations, it can be performed rapidly as it does not require much pre-procedure work up and is widely available in most tertiary care and trauma centers. Another advantage of MDCT angiography is that, once images have been properly acquired, the dataset can be explored any number of times to address many specific questions without any added procedure on the patient.
The accurate localization of these arteries prior to bronchial artery embolization or surgical intervention helps to shorten the procedure time and reduces complications. In various reports, it can be seen that bronchial artery aneurysms have been successfully detected with contrast-enhanced CT scan.[5–9] Clear depiction of vascular nature of these lesions on CT also helps to avoid percutaneous or bronchoscopic biopsy, which will have unforeseen consequences. In our case, MDCT angiography not only showed the parenchymal abnormality and enlarged bronchial arteries, but also depicted the small aneurysm apparently responsible for hemoptysis. Of the many available reconstruction algorithms, volume rendering (VR), and maximum intensity projection (MIP) are the most commonly employed techniques for obtaining angiographic-quality images from the axial CT data.[14] The number, size, course, and relationships of the bronchial as well as non-bronchial systemic arteries can be easily demonstrated by using real-time interactive editing of these reconstructions, and the vascular anatomy can be viewed from different perspectives by rotating these images. Coronal and coronal oblique images are very helpful; sometimes a curved planar reformation may be used to provide a visual summary of the entire vascular anatomy over a single image.
Bronchial and non-bronchial systemic artery embolization is an established procedure for the treatment of massive hemoptysis.[10] Massive hemoptysis due to pulmonary tuberculosis can also be effectively controlled with transcatheter embolization technique.[2] Intrapulmonary bronchial artery aneurysms have been successfully treated with transcatheter embolization; however, large aneurysms and those located in mediastinum may require surgery or aortic stent graft.[5–9] As bronchial artery embolization only treats the hemoptysis, the underlying disease process needs to be appropriately addressed to prevent recurrent hemoptysis.[10]
CONCLUSION
To conclude, we present an uncommon case of bronchial artery aneurysm due to pulmonary tuberculosis detected by MDCT angiography. MDCT angiography is useful for the correct diagnosis of the cause of hemoptysis and underlying vascular abnormality. In addition, CT angiographic findings enable direct selective catheterization and embolization of abnormal bronchial artery responsible for hemoptysis. MDCT angiography should be performed as initial investigation in all cases presenting with massive hemoptysis.
Source of Support: Nil,
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/26/81293
REFERENCES
- World Health Organisation Media centre: Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/
- [Google Scholar]
- Lessons from patients with hemoptysis attending a chest clinic in India. Ann Thorac Med. 2009;4:10-2.
- [Google Scholar]
- Massive hemoptysis due to pulmonary tuberculosis: Control with bronchial artery embolization. Radiology. 1996;200:691-4.
- [Google Scholar]
- Bronchial and nonbronchial systemic arteries at multi–detector row CT angiography: Comparison with conventional angiography. Radiology. 2004;233:741-9.
- [Google Scholar]
- Ruptured bronchial artery aneurysm associated with bronchiectasis: A case report. Ann Thorac Cardiovasc Surg. 2009;15:115-8.
- [Google Scholar]
- Bronchial artery aneurysm treated with aortic stent graft and fibrin sealant. Ann Thorac Surg. 2007;83:693-5.
- [Google Scholar]
- Ruptured bronchial artery aneurysm associated with sarcoidosis. Thorac Cardiovasc Surg. 2003;125:1153-4.
- [Google Scholar]
- Bronchial and nonbronchial systemic artery embolization for lifethreatening hemoptysis: A comprehensive review. Radiographics. 2002;22:1395-409.
- [Google Scholar]
- A 44-year-old man with hemoptysis: a review of pertinent imaging studies and radiographic interventions. Cleve Clin J Med. 2008;75:601-7.
- [Google Scholar]
- Multi-slice CT angiography: a practical guide to CT angiography in vascular imaging and intervention. Br J Radiol. 2004;77:27-38.
- [Google Scholar]






