Translate this page into:
Role of Secretin-Enhanced Magnetic Resonance Cholangiopancreatography in the Evaluation of Patients Following Pancreatojejunostomy
Address for correspondence: Dr. Munazza Anis, Department of Radiology, Medical University of South Carolina, 1479, Long Grove Drive, Apt 103, Mount Pleasant, SC 29464, USA. E-mail: anis@musc.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:

This study was conducted to assess the role of secretin-enhanced magnetic resonance cholangiopancreatography (S-MRCP) in the evaluation of patients following pancreatico-jejunal anatomosis.
Materials and Methods:
S-MRCP studies (n = 83) performed at Brigham and Women's Hospital between 1/2005 and 7/2005 were retrospectively reviewed. Among these, there were 13 patients (10 females, 3 males; mean age = 45 years, range = 18-74 years) who were evaluated with S-MRCP following pancreatojejunal anatomosis. Single-shot fast spin-echo T2-weighted thick slab dynamic MRCP images obtained before and every minute (for 10 min) after IV injection of secretin (2 mcg/kg body weight of SecreFloTM IV over 1 min) were reviewed retrospectively and independently by 3 readers. Image analysis included measurement of the main pancreatic duct (MPD) diameter and subjective assessment of the grade of visualization of the MPD remnant. The amount of jejunal fluid and visualization of the pancreatico-jejunal anatomosis pre-and post-secretin were also documented. Direct correlation with endoscopic retrograde cholangiopancreatography (ERCP) finding was available in six of the 13 cases.
Results:
The MPD diameter and MPD remnant visualization improved post-secretin for 1/3 readers. The number of pancreatico-jejunal anastomoses and the amount of jejunal fillings pre-and post-secretin was seen to improve significantly for 1 of the 3 readers. For Reader 1, the mean MPD diameter in the body of the pancreas, on the pre-and post-secretin image, was 3.2 ± 1.3 mm and 3.8 ± 1.9 mm, respectively. There was no statistical difference in the values pre- and post-secretin in the MPD diameter (P = 0.07), MPD visualization (P = 0.16) and the number of pancreatico-jejunal anastomoses seen (P = 0.125 5/13 pre- and 9/13 post-secretin). Statistical significance was seen in the amount of jejunal filling (P = 0.01) after secretin. For Reader 2, the MPD diameter pre-and post-secretin was 4 ± 2 and 3.9 ± 2.1 mm, respectively (P = 0.89). The MPD visualization (P = 0.19) and degree of jejunal filling (P = 0.7) did not improve significantly. There were 3/13 pancreatico-jejunostomy anastomoses seen pre- and 8/13 seen post-secretin (P = 0.06). The values for Reader 3 reached a statistical significance for the measurement of MPD (P = 0.032). In addition, MPD visualization (P = 0.038), the number of anastomoses seen (P = 0.016) and jejunal filling (P = 0.006) were also significantly improved.
Conclusion:
The addition of intravenous secretin to an MRCP study in the evaluation of patients following pancreatojejunal anastomosis does not significantly impact the visualization of the pancreatic duct. However, secretin may improve the assessment of the pancreatico-jejunal anastomosis.
Keywords
Magnetic resonance cholangiopancreatography
partial pancreatectomy
secretin
INTRODUCTION
Secretin-enhanced magnetic resonance cholangiopancreatography (S-MRCP) has been shown to improve image quality in patients with a normal or non-dilated main pancreatic duct (MPD).[1–4] However, its role in the management of patients with pancreatojejunal anastomosis is still evolving. Pancreatico-duodenectomy is among the most common surgeries performed for pancreatic pathology. Long-term complications associated with this type of surgery include pancreatic insufficiency and chronic pancreatitis. The latter is diagnosed based on both evaluations of the functional integrity of the gland and the typical morphologic changes in the pancreatic duct seen at endoscopic retrograde pancreatography (ERP). Ductal abnormalities detected at ERP, however, may not be closely related to the degree of pancreatic functional impairment. Discrepancies between morphology and function are found in 12-29% of cases.[3–6] Therefore, it is important to evaluate the function of the pancreatic remnant itself to optimize enzyme treatment and, in some cases, to enable monitoring of possible endoscopic or surgical procedures. Evaluation of remnant pancreatic function based on biochemical tests is fraught with problems because of dependency on extra pancreatic factors, such as intestinal disorders, hepatic function, and renal function.
Despite the fact that ERP remains the most sensitive and specific test currently available for visualization of the pancreatic duct, this technique is invasive, and may be associated with complications. Moreover, patients undergoing ERP typically need sedation. Other disadvantages include lack of information on possibly coexistent extraductal lesions and unsuccessful cannulation in 3-10% of the cases, even in large endoscopic centers. Inexperience of the endoscopist and anatomic alterations, such as previous gastric, enteric or pancreatic surgery, duodenal stenosis, or presence of a juxtaampullary diverticulum are known predisposing factors that lead to higher rates of unsuccessful ERP.
Matos et al.,[2] showed that magnetic resonance (MR) pancreatography after secretin stimulation is useful for the morphologic and functional evaluation of the pancreatic duct. Secretin administration stimulates fluid and bicarbonate secretion by the exocrine pancreas and induces a transitory increase in the diameter of the pancreatic duct, which improves visualization. In addition, the degree of duodenal filling resulting from the drainage of pancreatic fluid can be evaluated semi-quantitatively and used as an indirect measure of pancreatic exocrine function.
Secretin-enhanced MR pancreatography has been used to improve visualization of the pancreatic duct and to provide qualitative assessment of jejunal filling. Matos et al.,[4] have shown the utility of S-MRCP for diagnosing pancreatic papillary stenosis or dysfunction and for detecting reduced pancreatic exocrine reserve. We hypothesized that S-MRCP could be used as a non-invasive technique to evaluate patients following pancreatojejunal anastomosis.
Therefore, the objective of our study was to assess the role of S-MRCP in the evaluation of patients following a pancreatojejunal anastomosis.
MATERIALS AND METHODS
Subjects
This is a retrospective cohort study. S-MRCP of 13 patients who had undergone a pancreatojejunal anastomosis between January 2005 and August 2005 were obtained and included in this study. Ten patients were female and three were male with an age range of 28-74 years (mean = 47 years). Indications for S-MRCP included evaluation of the pancreatic duct (n = 9), pancreatic function (13), or assessment of local recurrence (n = 4). Institutional review board approved this retrospective study. Informed patient consent was waived.
Prior pancreatic surgeries included 10 Whipple, one Frey, one Puestow, and one central pancreatectomy procedures. Among the patients who underwent Whipple's procedure, indications included chronic pancreatitis involving the pancreatic head in five patients, intraductal papillary mucinous neoplasm in four patients, and autoimmune pancreatitis in one patient. Frey and Puestow procedures were performed for chronic pancreatitis involving the pancreatic body; the central pancreatectomy was performed for a small solid and papillary epithelial neoplasm.
Imaging technique
All magnetic resonance imaging (MRI) examinations were performed using a 1.5-T scanner (GE 1.5 Tesla Signa LX 9.1) with a phased-array surface eight-channel torso coil. Single-shot, fast spin-echo T2-weighted thick slab dynamic MRCP images TR (Time of Recovery) 1000/TR Min; BW (bandwidth) 41.67; FOV (field of view) 36 cm; echo-train length, 256; section thickness, 40 mm; matrix, 256 × 256; acquisition time, 2 s) were obtained before and every minute (for 10 min) after IV injection of secretin (2 mcg/kg body weight of SecreFloTM IV over 1 min). Axial fat-saturated 3D FAME (Fast Acquisition with Multiphase Efgre 3D) T1 weighted pre- and post-gadolinium images were also obtained (TE (time to echo) Min, TI 24, slice thickness 5 mm, 0.5 NEX (number of excitations) 128 × 256) in all patients.
Image analysis
All 13 S-MRCP studies were analyzed independently and retrospectively by three radiologists blind to all clinical, surgical, and radiological information. Reader 1 is a qualified abdominal imaging fellowship trained junior radiologist. Reader 2 is a fellow specializing in abdominal imaging, whereas Reader 3 is an experienced fellowship trained abdominal imaging radiologist with special interest in pancreatic pathology and MRI.
The pre-secretin evaluation included measurement of the diameter of the MPD in millimeters in the region of the body and tail. The grades of visualization of the MPD were defined as follows: 1 = poor (the anatomic part was difficult to detect or only minimally visible), 2 = fair (the anatomic part was mostly visible), 3 = good (the entire anatomic part was visible), or 4 = excellent (the entire anatomic part was clearly visible). The jejunal filling volume was semi-quantitatively evaluated at 10 min and was graded as follows, according to the method described by Matos et al.,[4] Grade 1 (no secretion or filling limited to the anastomotic jejunal loop), Grade 2 (filling between first and second jejunal loops), and Grade 3 (filling of the first two jejunal loops or more). The pancreatico-jejunal anastomosis was documented as identified or as not seen.
Similar evaluation was performed for the post-secretin MRCP studies, which were reviewed during a separate session (interval > 4 weeks).
Statistical analysis
The differences in the diameter of the MPD between pre- and post-secretin images were analyzed using Student's paired t-test. The differences in the grades of visualization of the MPD and jejunal filling between pre-and post-secretin images were analyzed using Wilcoxon matched pairs test. The visualization of the pancreatico-jejunal anastomosis on the pre-and post-secretin image was compared using McNemar test. The inter-observer agreement for qualitative data was analyzed using unweighted (visualization of anastomoses) or linear weighted (grades of jejunal filling and visualization of MPD) kappa statistic. In general, a kappa value greater than 0.80 is considered excellent agreement; value between 0.61 and 0.80, good agreement; value between 0.41 and 0.60, moderate agreement; value between 0.21 and 0.40, fair agreement; and value of 0.20 or less, poor agreement.[7]
RESULTS
For Reader 1, pre- and post-secretin MPD diameter in millimeters in the pancreatic body equal 3.2 ± 1.3 and 3.8 ± 2. MPD visualization was 2.4 ± 1.8 and 2.6 ± 1.1 pre- and post-secretin. The jejunal filling was 1.4 ± 0.7 pre- and 2.3 ± 0.9 post-secretin. Of the 13 patients, 5/13 pancreatico-jejunal anastomoses were identified by pre-secretin images and 9/13 post-secretin images. There was no significant improvement in the MPD diameter in the pancreatic body (P = 0.07), MPD visualization (P = 0.16), or in the number of anastomoses seen (P = 0.125) pre- and post-secretin. The degree of jejunal filling improved pre- and post-secretin (P = 0.02) as shown in Table 1 [Figures 1 and 2].
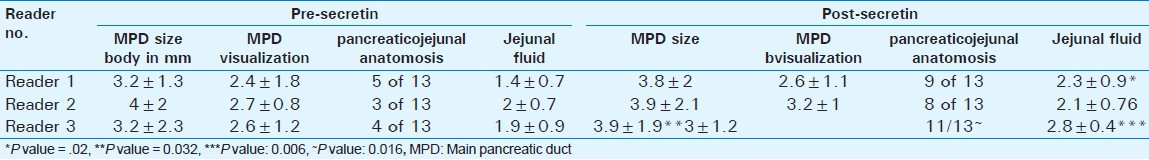

- Post-Whipple anatomy on magnetic resonance cholangiopancreatography. Pre-secretin MRCP image in a patient post-Whipple shows mildly dilated pancreatic duct (arrow head) and the pancreato-jejunostomy. Dashed arrow delineates the choledocho-jejunostomy.
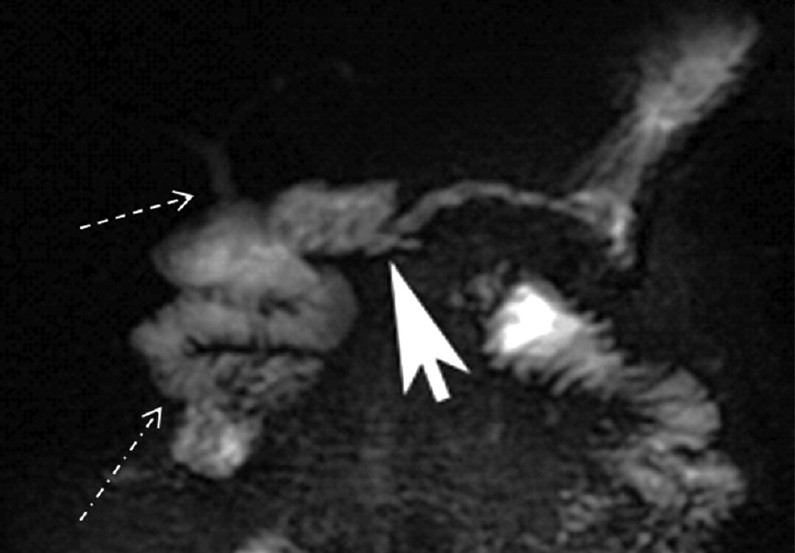
- Post-secretin magnetic resonance cholangiopancreatography of a Whipple patient. Post-secretin MRCP image shows no significant change in the size of the main pancreatic duct but improved visualization of the pancreatico-jejunostomy (arrow head). Delineation of the choledochojejunostomy is also improved (dashed arrow). Dash-dot arrow shows increased fluid in the proximal jejunum.
For Reader 2, the mean MPD diameter in the pancreatic body was 4 ± 2 and 3.9 ± 2.1 mm. MPD visualization was not significantly improved pre-and post-secretin (2.7 ± 0.8 and 3.2 ± 1, respectively) [Figures 3 and 4]. Of the 13 patients, 3/13 anastomoses were seen in pre-secretin scans and 8/13 in post-secretin scans. Jejunal filling pre-and post-secretin was 2 ± 0.7 and 2.1 ± 0.76. There was no statistical difference in the values pre-and post-secretin; MPD diameter (P = 0.89), MPD visualization (P = 0.19), and jejunal filling (P = 0.07). The pancreatojejunal anastomosis seen pre- and post-secretin improved appreciably, however, not to a statistical significance (P = 0.07).
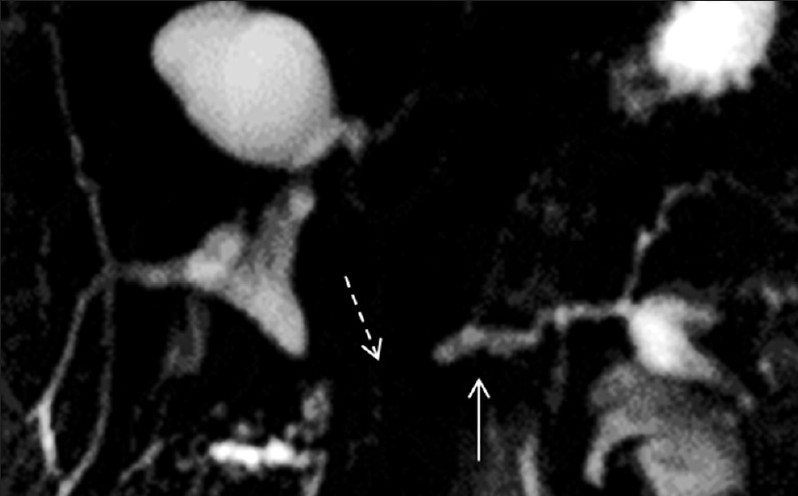
- Pre-secretin magnetic resonance cholangiopancreatography in a patient with pancreato-jejunostomy. The main pancreatic duct is mildly dilated (arrow) with non-visualization of the pancreato-jejunostomy (dashed arrow).

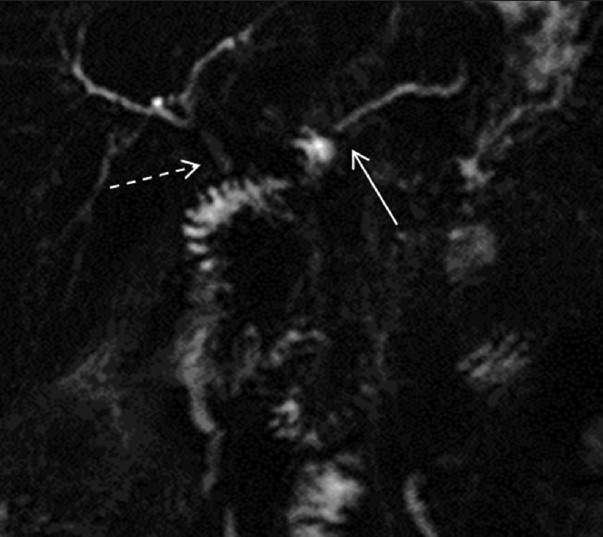
- Post-secretin magnetic resonance cholangiopancreatography (MRCP) in a patient with pancreato-jejunostomy. MRCP images show improved visualization of the pancreato-jejunostomy anastomosis (dashed arrow) but no change in the main pancreatic duct size (arrow).
For Reader 3, the MPD diameter in the body was 3 ± 2.3 pre-secretin and 3.9 ± 1.9 mm post-secretin. MPD visualization was 2.6 ± 1.2 and 3 ± 1.2 pre- and post-secretin. Of the 13 patients, 4/13 anastomoses were seen before secretin and 11/13 were seen after secretin images. Jejunal filling was 1.9 ± 0.9 and 2.8 ± 0.4 pre- and post-secretin [Figures 5 and 6]. There was a statistical significance in the measurement of MPD diameter in the body (P = 0.032), MPD visualization (P = 0.038), the number of anastomoses seen (P = 0.016), and jejunal filling (P = 0.006), pre- and post-secretin.
- Pre-secretin magnetic resonance cholangiopancreatography Whipple. The image shows pancreato-jejunostomy (arrow) and choledocho-jejunostomy (dashed arrow).

- Post-secretin magnetic resonance cholangiopancreatography in a Whipple patient. The pancreatic (arrow) and choledochal anastomosis (dashed arrow) visualization is improved. In addition, marked increase in the fluid (dotted arrow) is noted in the proximal jejunal segments.
The inter-observer agreement for Reader 1-2, Reader 1-3, and Reader 2-3 pre- and post-secretin was mostly > 0.5 for jejunal filling, MPD visualization, and anastomoses. However, for Reader 2-3 agreement was 0.06 for jejunal filling and Reader 1-3 agreement was 0.16 for number of anastomoses seen post secretin.
ERCP was available for 6/13 patients. The ERCP and MRCP showed similar findings in four of the six cases [Figure 7]. ERCP and MRCP each misdiagnosed stenosis of pancreatojejunal anatomosis for different patients; however, clinical data, including biochemical evaluation and follow-up studies suggested, otherwise. One patient developed pancreatitis following ERCP.
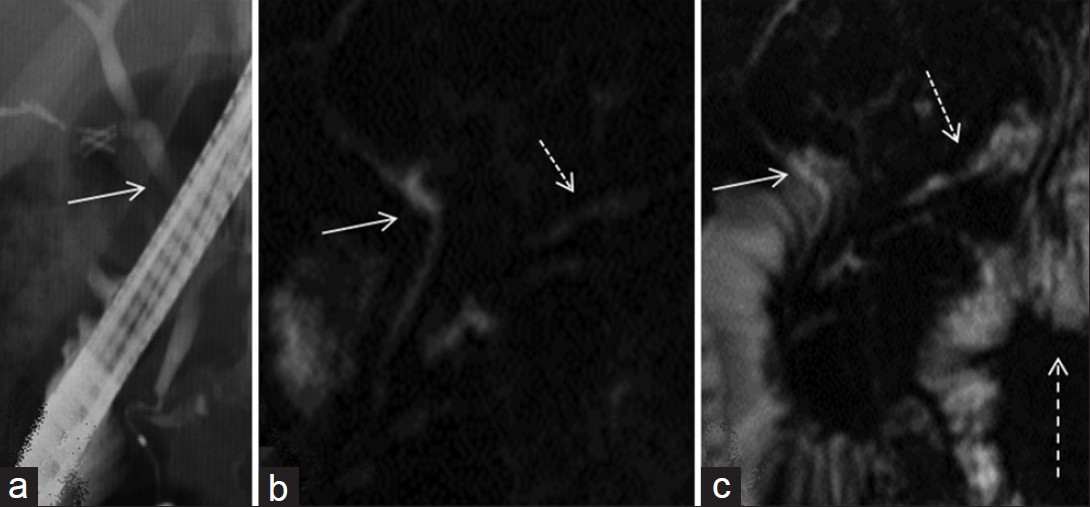
- (a) ERCP and magnetic resonance cholangiopancreatography (MRCP) images (b) before and (c) after secretin. (a) normal endoscopic cholangiogram in a patient with lateral pancreto-jejunostomy. (b) MRCP images demonstrate findings of a normal biliary system (arrow) with a lateral pancreato-jejunostomy (dotted arrow). (c) visualization is improved after secretin (dashed arrow). Note increased jejunal fluid after intravenous secretin (dashed arrow).
Most patients in our study demonstrated slow rise (2 min after the IV gadolinium injection) in pancreatic enhancement and slow decline compatible with pancreatic fibrosis [Figure 8].
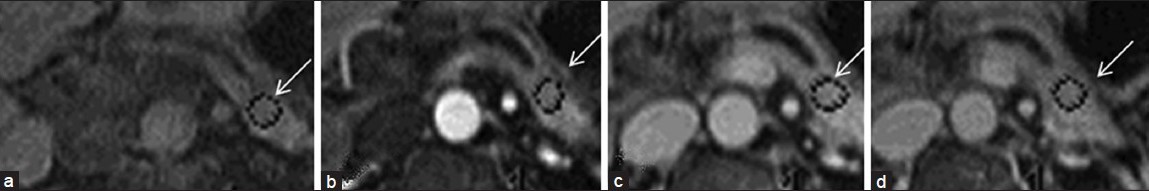
- Pre- and dynamic post- gadolinium images of a patient with chronic pancreatitis. (a) Non-contrast image shows low T1 signal intensity within the pancreas (arrow) compatible with chronic pancreatitis. Subsequently (b, c), there is a slow rise to peak enhancement. (d) delayed phase demonstrates a slow washout within the pancreas compatible with pancreatic fibrosis.
DISCUSSION
This study brings to attention several important factors relating to the use of intravenous (IV) secretin in patients with pancreatojejunal anastomosis, which is associated with 30-50% of pancreatic parenchymal loss, with consequent reduction in pancreatic secretion. Pancreatic insufficiency and atrophy of the pancreatic parenchyma is likely related to post-surgical alteration of pancreatic neurohormonal stimulator mechanisms or to stenosis of the pancreatojejunal anastomosis. Chronic pancreatitis is another complication that is frequently encountered in patient's post-pancreatic surgery with ensuing abnormalities of the pancreatic duct, where IV secretin has been seen to have a role.[89] Time intensity curve obtained from dynamic MRI has been shown to be a reliable indicator of fibrosis in the remnant pancreas after pancreatico-duodenectomy.[10] Duct to mucosa anastomosis is associated with a lower risk of pancreatic fibrosis 1-3 years after surgery than a pancreatojejuno-serosal anastomosis. Normal pattern of pancreatic enhancement is characterized as rapid rise to a peak (25 s after 10 cc intra venous gadolinium injection) followed by a rapid decline. Slow rise to a peak (1 min after the IV gadolinium injection) and slow decline is defined as pancreatic fibrosis.
Post-operative follow-up for pancreatic morphology and function is important for optimizing medical treatment and for eventual decision-making about the use of more aggressive (endoscopic or surgical) therapeutic procedures. Sonography and computed tomography (CT) are not sufficiently sensitive for the early detection of changes in ductal morphology or incipient grades of atrophy.
We did not see a consistent improvement in the MPD diameter or visualization; however, various studies have shown improvement in the image quality of the pancreatic duct in MR pancreatography with secretin stimulation,[3–6] with results similar to those obtained by ERCP. Patients who have undergone pancreatoduodenectomy, however, lack sphincter of Oddi mechanism, which is one of the factors responsible for transient dilatation of the MPD. Other factors may include lack of distensability and baseline dilatation of the duct in the presence of chronic pancreatitis. Lack of response to secretin in the presence of ductal strictures or diameter greater than 5 mm has been shown by several authors, including Hellerhoff et al.[11] In addition, loss of exocrine function is thought to precede morphologic changes in the duct in chronic pancreatitis with no direct relationship between jejunal filling grade and exocrine function.
The patency of pancreaticojejunostomy anastomosis is of paramount importance in conserving the exocrine function of the pancreas in patients who have undergone partial pancreatectomy. Stenosis of the surgical anastomotic site is associated with post-obstructive chronic pancreatitis and pancreatic atrophy. Early diagnosis is, therefore, needed to allow for immediate endoscopic or surgical treatment. In our data, addition of secretin to an MRCP study led to significant improvement in visualization of the anastomotic site. Statistical significance is also evident for jejunal filling post-secretin, which indicates relative preservation of pancreatic function in our patient population. ERCP obtained in half of our patients rendered the same information as S-MRCP, however, led to acute pancreatitis in one case. In a study by Czako et al.,[12] the value of MRCP was assessed in the management of patients in whom ERCP was unsuccessful. MRCP prevented an invasive procedure in 15 of 22 cases and guided therapy in the remaining seven patients.
The limitations of our study include retrospective study design and lack of direct correlation with ERCP in all patients. Different degrees of experience of our readers also brings to attention the importance of increasing familiarity with these studies as their use is becoming more widespread. Variability of measurements between the three readers calls for an electronic program in clinical practice to overcome the individual differences.
CONCLUSION
Our study emphasizes the importance of S-MRCP in the diagnosis and management of pancreatojejunal anastomsis patients. It is safe and non-invasive. In addition to the MPD evaluation, there are other clinical information to be gleaned from this exam regarding pancreatic exocrine function and the patency of the anastomotic sites that significantly impact patient management.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/7/107909
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Severe chronic pancreatitis versus suspected pancreatic disease: Dynamic MR cholangiopancreatography after secretin stimulation. Radiology. 2000;214:849-55.
- [Google Scholar]
- Pancreatic duct after pancreatoduodenectomy: Morphologic and functional evaluation with secretin-stimulated MR pancreatography. AJR Am J Roentgenol. 2004;183:1267-74.
- [Google Scholar]
- Pancreatic duct: Morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435-41.
- [Google Scholar]
- Pancreatic duct: Morphologic evaluation with MR cholangiopancreatography after secretin stimulation. Radiology. 2002;222:674-80.
- [Google Scholar]
- Chronic pancreatitis: Evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology. 2000;215:358-64.
- [Google Scholar]
- The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-74.
- [Google Scholar]
- Complications after pancreatoduodenectomy: Imaging and imaging-guided interventional procedures. RadioGraphics. 2001;21:673-90.
- [Google Scholar]
- Dynamic magnetic resonance imaging to evaluate remnant pancreatic fibrosis. Br J Surg. 2004;91:595-600.
- [Google Scholar]
- Dynamic MR pancreatography after secretin administration: Image quality and diagnostic accuracy. AJR Am J Roentgenol. 2002;179:121-9.
- [Google Scholar]
- Diagnostic role of secretin-enhanced MRCP in patients with unsuccessful ERCP. World J Gastroenterol. 2004;10:3034-8.
- [Google Scholar]






