Translate this page into:
Nonfunctional Islet Cell Tumor of the Pancreas in a Patient with Tuberous Sclerosis: A Case Report with Literature Review
Address for correspondence: Dr. Aysegul Cansu, Department of Radiology, Faculty of Medicine, Karadeniz Technical University, Farabi Hospital, 61080 Trabzon, Turkey. E-mail: drcansu@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Islet cell tumors (ICTs) are rare tumors of the pancreas. Association of this type of tumor with tuberous sclerosis is extremely rare. Only 13 cases of pancreatic ICT with tuberous sclerosis have so far been documented in the literature. However, awareness of the association of tuberous sclerosis and ICT is important for early diagnosis and appropriate treatment of this condition. This article presents the case of a 63-year-old female with angiomyolipoma (AML) of the kidney and liver, calcified subependymal nodules and a large mass in the pancreas, which was proven to be an ICT on histopathological examination.
Keywords
Angiomyolipoma
islet cell tumor
multislice computed tomography
pancreas
tuberous sclerosis
INTRODUCTION

Tuberous sclerosis is a rare multisystem disorder characterized by widespread hamartomas in several organs including the brain, heart, skin, eyes, lungs, kidneys, liver, and bones.[1] The most commonly involved abdominal organ is the kidney, which is affected in up to 80% of all cases. The most common lesion, in these cases, is an angiomyolipoma (AML).[2] Involvement of the liver tends to be less frequent than that of the kidneys and is therefore less well-documented.[3]
An islet cell tumor (ICT) is a rare neoplasm of the pancreas accounting for 1%-3% of all pancreatic tumors,[4] although an association with tuberous sclerosis is extremely rare it can occur. To our knowledge, only 13 cases of pancreatic ICT with tuberous sclerosis have been reported.[567] We are presenting a case of a 63-year-old-woman with AML of the kidney and liver, calcified subependymal nodules, and a large mass in the pancreas, which was identified histopathologically to be an ICT.
CASE REPORT
A 63-year-old-woman was referred to our department with a complaint of abdominal pain. Upon physical examination, skin lesions were detected and a firm mass was palpable in the upper quadrant of the abdomen. Laboratory findings were unremarkable. She was referred to the radiology department for a computed tomography (CT) examination of the abdomen. A biphasic contrast enhanced CT was also performed on a 16-slice CT system (Somatom Sensation, Siemens, Erlangen, Germany). An intravenous injection of 130 mL contrast media (iopamidol with an iodine concentration of 300 mg/mL) was given at a rate of 4 mL/s. Scanning was initiated after a delay of 25 s and 70 s after infusion of contrast material for the arterial phase and venous phase images, respectively. Scanning parameters were as follows: Slice collimation, 16 × 1.5 mm; table feed/rotation, 18.0 mm; rotation time, 0.5 s; KV 120; effective mAs 160, slice thickness, 2 mm.
CT examination revealed a 9 × 6 cm mass with discrete nodular calcifications in the head of the pancreas. The mass showed heterogeneous enhancement in the arterial phase and homogenous enhancement in the venous phase [Figure 1a and b]. The gastroduodenal artery was seen encased by the pancreatic mass. There were also multiple lesions in the liver ranging in diameter from 3 to 25 mm. Some of the lesions showed homogenous fat-density; however, others had heterogeneous areas of soft-tissue density and fat-density that were suggestive of AML. Lesions with homogenous fat-density did not show any enhancement in both the arterial and the venous phase images [Figure 2]. In lesions containing both a soft tissue component and a fat component, the nonfat component was enhanced markedly in the arterial phase and showed isohypodensity in the venous phase. Four lesions ranging from 3 to 12 mm in diameter had no fat components. These lesions had homogenous enhancement in the arterial phase image and became isohypodense in the venous phase image [Figure 3]. There were also multiple fat-containing lesions in both kidneys compatible with AML [Figure 1b] as well as multiple small discrete sclerotic lesions in the vertebrae. A cranial CT revealed multiple calcified subependymal nodules [Figure 4]. Unfortunately, as the patient was claustrophobic she did not undergo a magnetic resonance imaging (MRI) investigation.
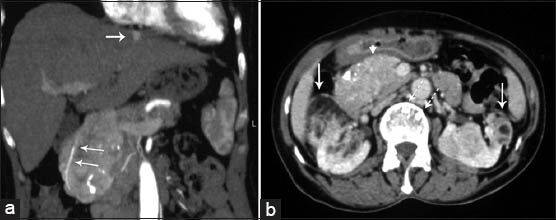
- 63-year-old woman with abdominal pain diagnosed with angiomyolipoma (AML) of the kidney and liver, and islet cell tumor of the pancreas. Contrast-enhanced computed tomography (a) Coronal reconstruction image obtained during the arterial phase of enhancement shows a large heterogeneously enhanced mass. Gastroduodenal artery is encased by the mass (arrows). Hyperenhancing lesion in the liver is also seen (arrow). (b) Axial image obtained in the venous phase of enhancement shows that the mass enhanced homogenously and had calcifications (arrow head). There are bilateral renal AMLs containing fat density (arrows). Note also discrete vertebral sclerotic lesions (dashed arrow).
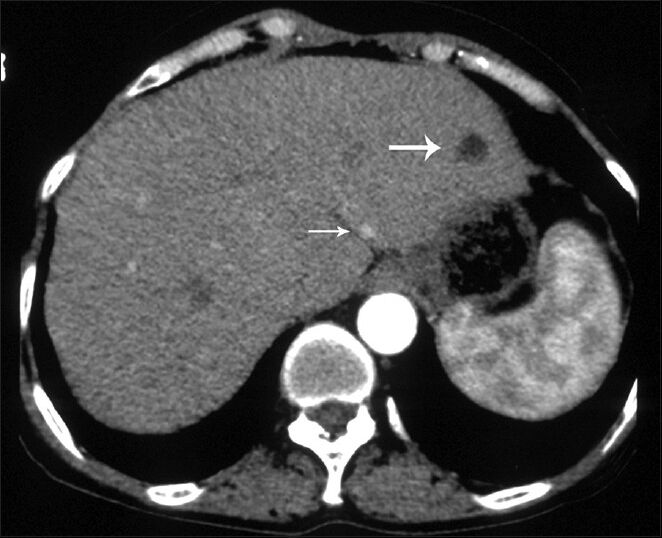
- 63-year-old woman with abdominal pain diagnosed with angiomyolipoma (AML) of the kidney and liver, and islet cell tumor of the pancreas. Axial image obtained in arterial phase shows an AML in segment 2 of the liver containing homogenous fat density without enhancement (large arrow) and hyperenhancing small lesion (small arrow).
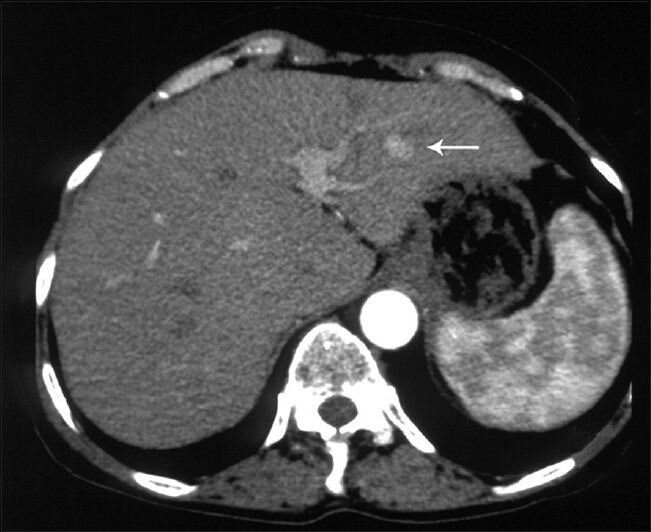
- 63-year-old woman with abdominal pain diagnosed with angiomyolipoma of the kidney and liver, and islet cell tumor of the pancreas. Axial image obtained in the arterial phase shows a hyperenhancing lesion in segment 3 of the liver (arrow).
The diagnosis of tuberous sclerosis was established on the basis of radiologic findings of renal and hepatic AML, calcified subependymal nodules, and vertebral sclerotic lesions with a clinical finding of adenoma sebaceum. Ultrasound-guided fine-needle aspiration of the pancreatic mass was performed using a 20-gauge needle. Histological examination of the cell-block preparations of the mass showed solid sheets of neuroendocrine-appearing cells characterized by large eosinophilic cytoplasm with moderate nuclear pleomorphism [Figure 5]. Immunochemical staining showed the cells were positive for synaptophysin and a few tumor cells were positive for chromogranin. On the basis of the morphologic and immunohistochemical findings, the lesion was diagnosed as ICT. Since the patient's serum levels of the pancreatic hormones were within the normal limits, ICT was considered as a nonfunctioning lesion.
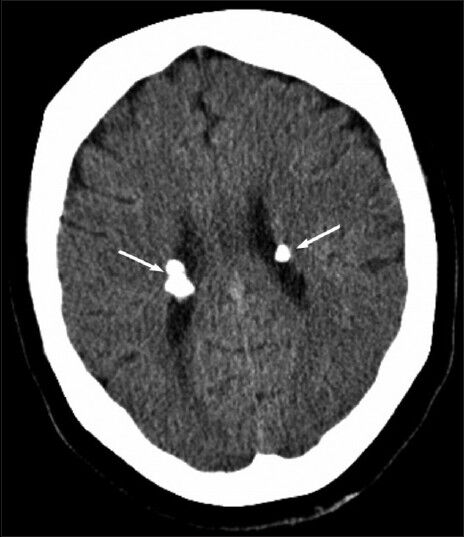
- 63-year-old woman with abdominal pain diagnosed with angiomyolipoma of the kidney and liver, and islet cell tumor of the pancreas. Axial computed tomography image of the brain shows calcified subependymal nodules (arrows).
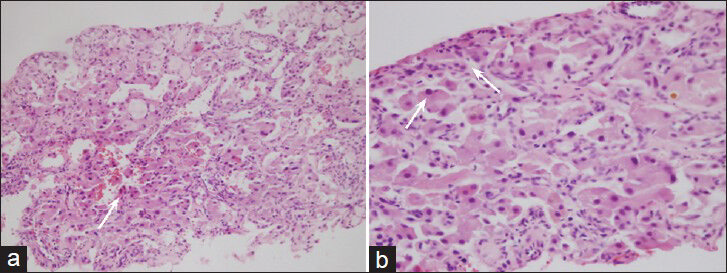
- 63-year-old woman with abdominal pain diagnosed with angiomyolipoma of the kidney and liver, and islet cell tumor of the pancreas. H and E, stained specimen (a) ×200 and (b) ×400 show neoplastic cells forming solid sheets and also have large eosinophilic cytoplasm with moderate nuclear pleomorphism (arrows). Some neoplastic cells show binucleation.
DISCUSSION
Tuberous sclerosis is a rare autosomal dominant disorder, which can affect almost all body systems. It is caused by the mutation of two tumor suppressor genes, TSC1 and TSC2, which encode hamartin and tuberin, respectively.[1] The tuberin-hamartin complex has been shown to be critical for controlling cell growth and proliferation. The majority of the legions in tuberous sclerosis are benign hamartomas, which rarely become malignant. Tuberous sclerosis may affect the central nervous system (cortical tubers, subependymal nodules, and astrocytomas), skeletal system (sclerotic and lucent bone lesions), skin (sebaceous adenomas, and fibromas), eyes (retinal hamartomas), lungs (lymphangioleiomyomatosis), heart (rhabdomyomas), and abdominal organs (AMLs and cysts).[7] The most commonly affected abdominal organs are the kidneys, which are affected in up to 80% of all reported cases. AML is the most common lesion,[2] followed by renal cysts. Cysts may be bilateral or unilateral lesions. Initially, the cysts tend to be small in size and fewer in number but over time there is a tendency for the cysts to increase in size and number.[2] Renal cell carcinoma or oncocytoma may also be seen. The involvement of the liver in tuberous sclerosis is much less common and only 13% of patients with tuberous sclerosis were reported as having AML in the liver.[3] Pancreatic involvement in the form of cysts, AML, or ICT is very rare in tuberous sclerosis.
ICTs are rare with an annual incidence of <0.4 per 100,000 cases and accounts for only 1%-3% of all neoplasm of the pancreas.[4] Pancreatic ICTs can be sporadic or associated with a genetic syndrome. Genetic syndromes associated with ICTs include multiple endocrine neoplasia Type 1, von Hippel-Lindau disease, von Recklinghausen disease, and tuberous sclerosis. ICTs are very rarely reported with tuberous sclerosis. As noted previously, to our knowledge, only 13 cases of tuberous sclerosis in patients have been reported where ICT was present.[567] Five of these cases had insulinoma, one case had gastrinoma, one case had glucagonoma, one case had somatostatinoma, and the remaining five cases showed the presence of nonfunctional ICT.
ICTs are divided into functional and nonfunctional tumors. Functional tumors are classified based upon the hormones they produce as well as by the associated endocrine syndrome. The diagnosis of functioning ICTs is almost always established biochemically when the tumor is small in size. Nonfunctioning ICTs, that represent approximately one-half of all documented ICTs, usually appear at an advanced stage as either benign or malignant. When benign, the ICT shows as a large pancreatic mass and when malignant, extensive hepatic, pulmonary or lymph node metastasis is present. Nonfunctional ICTs show a high rate of malignancy, ranging from 60% to 92%.[8] Histological differentiation between benign and malignant tumors is difficult because tumors that metastasize are not necessarily histologically different from nonmetastasizing tumors. Tumors that are larger in size (>5 cm) and show evidence of necrosis, discrete nodular calcification and invasion of retroperitoneal structures can all indicate malignancy. The presence or absence of metastases is a major predictor of survival of the patient. In the presence of liver metastasis, patients may benefit from surgical resection or chemoembolization. However, in the presence of tuberous sclerosis, it may not always be possible to differentiate between hepatic metastasis of ICT and the hepatic AML.
The classic and most common enhancement feature of nonfunctional ICTs seen on contrast-enhanced CT is hyperattenuating lesions in the arterial phase, which is evident because of their rich vascular supply. CT findings of pancreatic ICT are similar to hypervascular metastasis, pancreatic hamartoma, acinar cell carcinoma, solid serous adenoma, and solitary fibrous tumors.[9] It has been suggested that pancreatic hamartomas can be seen with tuberous sclerosis; however, to our knowledge, a direct association has not been reported in the literature. Liver metastases of ICTs are also usually hypervascular and appear as hyperattenuating masses in the arterial phase images on CT. A diagnosis of hepatic AML can be made upon detection of an unenveloped nodule with intratumoral fat, prominent central vessel, and no capsule.[3] Definitive preoperative diagnosis of AML, especially those with lipid deficient characteristics, is a challenge for radiologists. In recent reports, there has been an increasing frequency of fat-deficient hepatic AML being present along with tuberous sclerosis. Dilated intratumoral vessels are less commonly detected in AML due to their small size in tuberous sclerosis.[10] Fat-deficient hepatic AMLs have been reported as having homogeneous-heterogeneous enhancement in arterial phase contrast-enhanced CT images.[10] It is difficult to differentiate fat-efficient hepatic AML from metastasis of ICT in tuberous sclerosis because of similar enhancement characteristics of these two lesions. Our patient had four lesions without any fat component in the liver, ranging from 3 to 12 mm that showed homogeneous enhancement in the arterial phase and became isodense in the venous phase. No biopsy was performed because of their small size. These four lesions were identified as fat-deficient AML, because the number and size of these lesions had not changed when a CT scan was done 6 months later.
CONCLUSION
In our case, a diagnosis of tuberous sclerosis was established very late, unlike in most cases reported in the literature that are diagnosed during childhood. After a diagnosis of tuberous sclerosis has been established, renal ultrasonography every 1-3 years is recommended. It should be noted that because of the high rate of malignancy, the pancreas should also be evaluated carefully during ultrasonography. Contrast-enhanced ultrasonography or endoscopic ultrasonography may also be performed for increasing the sensitivity and abdominal CT/MRI can be added as the next step. Being aware of the association between tuberous sclerosis and ICT is particularly important for early diagnosis and a favorable therapeutic outcome.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/3/126022
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Pancreatic endocrine neoplasms: Epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409-27.
- [Google Scholar]
- Pancreatic somatostatinoma and tuberous sclerosis: Case report of an exceedingly rare association. Gastrointest Endosc. 2009;69:379-81.
- [Google Scholar]
- Neuroendocrine tumor of the pancreas in a patient with tuberous sclerosis: A case report and review of the literature. Int J Surg Pathol. 2012;20:390-5.
- [Google Scholar]
- Well-differentiated pancreatic neuroendocrine carcinoma in tuberous sclerosis: Case report and review of the literature. Am J Surg Pathol. 2012;36:149-53.
- [Google Scholar]
- Nonfunctioning malignant neuroendocrine tumors of the pancreas. Surgery. 1986;100:978-88.
- [Google Scholar]
- Pancreatic imaging mimics: Part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am J Roentgenol. 2012;199:309-18.
- [Google Scholar]
- Hepatic angiomyolipoma: Dynamic computed tomography features and clinical correlation. World J Gastroenterol. 2009;15:3417-20.
- [Google Scholar]






