Translate this page into:
Evaluation of Quantitative and Qualitative Renal Outcome Following Nephron Sparing Surgery
Address for correspondence: Dr. Gautam Ram Choudhary, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India. E-mail: gautamoshu@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose:
Preservation of renal function after nephron-sparing surgery (NSS) is multifactorial and the impact of individual factors on it is still a debate. This prospective study investigates the impact of factors responsible for quantitative and functional outcome after NSS.
Patients and Methods:
Fifty-two patients of localized renal mass (≤7 cm) were included in the study. A contrast-enhanced computed tomography abdomen was performed for characterization of tumor. Glomerular filtration rate (GFR) was calculated using Tc99m-diethylenetriamine pentaacetic acid (DTPA) scan and Cockcroft-Gault (CG) formula. All relevant intra- and peri-operative events were noted. Follow-up work up performed at 3 months.
Results:
Overall, the mean ischemia time was 30.6 min, with 7.7% decrease in renal volume in the operated moiety. In follow-up, the total and ipsilateral GFR decreased. Change in renal parenchymal volume, total GFR by CG and DTPA, split GFR of tumor-bearing moiety was significant in follow-up. Size, stage, polar location of tumor, duration of surgery, type of ischemia, preoperative chronic kidney disease, and need of blood transfusion did not affect change in renal volume and function in the follow-up period.
Conclusion:
Renal parenchymal loss and duration of ischemia have impact on the follow-up renal function.
Keywords
Ischemia
nephrectomy
parenchymal
partial
renal

INTRODUCTION
The fundamental goal of nephron-sparing surgery (NSS) is to preserve renal function without compromising the oncologic outcomes. The ubiquitous use of, and improvement in, abdominal imaging modalities has resulted in increased detection of incidental renal masses that now account for 50%–65% of all diagnosed renal masses. These renal tumors are smaller, less likely to metastasize and hence often amenable to NSS. During the past 10–15 years, NSS has emerged as the gold standard for the treatment of small renal masses with equivalent oncological outcomes, better preservation of renal function, and improved overall survival compared with radical nephrectomy (RN). NSS has supplanted RN as the treatment of choice for T1 renal tumors when feasible, even in the absence of identifiable renal insufficiency.[1234]
Recent studies indicate that long-term renal dysfunction is more common following RN which is associated with coronary artery disease and death, hence, where ever plausible NSS should be performed. However, this comes at the cost of increased risk of complications such as hemorrhage, urinary fistula formation, ureteral obstruction, acute renal insufficiency, and infection.[5] The factors affecting the loss of renal function after NSS are different in different studies. It is logical to think that loss of renal function following NSS is multifactorial which includes clinical, surgical, patient, and surgeon factors. The individual impact and the relative potential contribution that each factor provide for the final functional outcome is still a gray zone and has not been well studied. The current literature also has suggested the functional outcome of the kidney is determined by the amount of preserved renal parenchyma and not on the size of the tumor.[23] After NSS function of remaining renal mass can be assessed by serum creatinine, creatinine clearance (CrCl) by 24-h urine collection, contrast-enhanced computed tomography (CT) with CT GFR (estimation of GFR by enhancing renal volume), estimated GFR (e-GFR) by CG formula, GFR from using the abbreviated modification in diet and renal disease study equation, and Technetium Tc 99m-diethylenetriaminepentaacetic acid (Tc 99m-DTPA) renal scintigraphy. After NSS, remaining renal parenchyma may be measured by various means, for example, ultrasound, CT with volumetric measurement or magnetic resonance imaging.[4678]
The present prospective study was aimed to evaluate the impact of various factors responsible for quantitative (as assessed by CT scan) and functional outcome (as assessed on DTPA scan/e-GFR) of NSS, at a short-term follow-up of 3 months.
PATIENTS AND METHODS
-
Study design and patient characteristics: This was a prospective study, conducted from July 2012 to May 2014. All patients of the localized renal mass of size up to 7 cm were eligible. The study was approved by Institute's Ethics Committee, and all patients were explained in detail about the study protocol. An informed written consent was obtained from those willing to participate in the study. Patients not willing to participate, radiological evidence of locally advanced and metastatic disease, dialysis-dependent chronic kidney disease (CKD), allergy to intravenous contrast agents, or contraindications for contrast-enhanced computed tomography (CECT) were excluded from the study. Flowchart is given for the study design [Figure 1]
-
Preoperative workup: A triphasic CECT of abdomen was performed for characterization of tumor. CT images in venous phases were analyzed using a real-time interaction approach on a dedicated syngo through (Siemens healthcare global) workstation with 3D rendering software [Figure 2]. N-acetyl cysteine 600 mg twice a day was administered 2 days before CECT and continued for one day after the procedure in a patient with raised serum creatinine (S. Cr.) level (>1.2 mg/dl). Total and split GFR using Tc99 m DTPA scan was performed either before CECT scan or at least 2 weeks later
-
Estimation of renal function: GFR was calculated by (1). CG formula = ([140-age] × [body weight in kg]) ÷ ([serum creatinine] × [72]) × (0.85) (female) and (2). 99Tc DTPA scan: Visual evaluation of the scintigraphy images was performed on the computer screen, then using Gates method (quantitative method) total and split GFR were estimated [Figure 3]. A postoperative change in GFR of >5% over the baseline value was considered significant[9]
-
Perioperative outcome assessments: After complete workup and fitness for surgery, patients were taken for surgery under general anesthesia. In perioperative period, adequate hydration was maintained by measuring hourly urine output and nephrotoxic drugs were avoided. Standard protocol of laparoscopic/open NSS was followed. Tumor resection was carried out in following steps (1) marking by monopolar diathermy, (2) resection of tumor with or without the help of intraoperative sonography, and (3) renorrhaphy in two layers with polyglactin 2–0 RB needle, calyceal repair separately if require with 3–0 polyglactin RB needle. 1 g/kg of 20% mannitol was administered by i.v., infusion 10–15 min before clamping renal vessels. Intraoperative hypothermia (whenever necessary) was achieved by surface cooling by ice slice for 10–15 min immediately after occlusion of the renal artery. Intraoperative parameters such as ischemia time, duration of surgery, blood loss, need of blood and blood product transfusion, urine output, and postoperative complications such as fever, bleeding, and urine leak were noted
-
Follow-up: Patients were followed up at 1 and 3 months. At 3 months’ postsurgery, they underwent serum creatinine, CECT abdomen, and DTPA scan
-
Measured outcome: Decreased renal volume of operated kidney by CECT, change in total GFR by CG formula, change in total and split GFR by DTPA method, effect of ischemia on these parameters and local or distant recurrence by CECT scan at 3 months’ follow-up, were measured in this study.
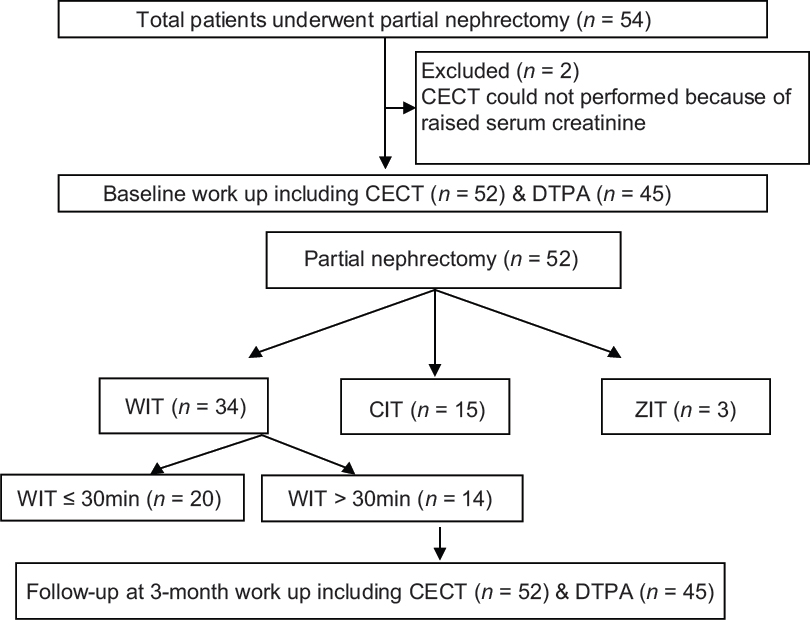
- Flow chart.
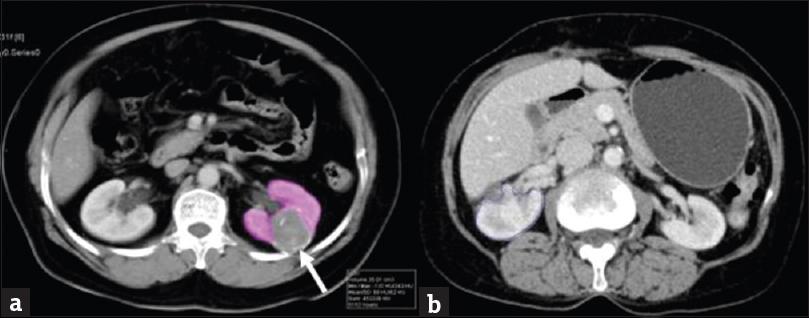
- Contrast-enhanced computed tomography of patient with renal mass. (a) Contrast enhancing computed tomography image at the level of kidney shows well defined hypoenhancing mass (arrow) at mid pole of the left kidney with volume estimation of rest of the normal kidney. (b) Contrast enhancing computed tomography image at the level of kidney shows well enhancing renal parenchyma of the right kidney following partial nephrectomy.
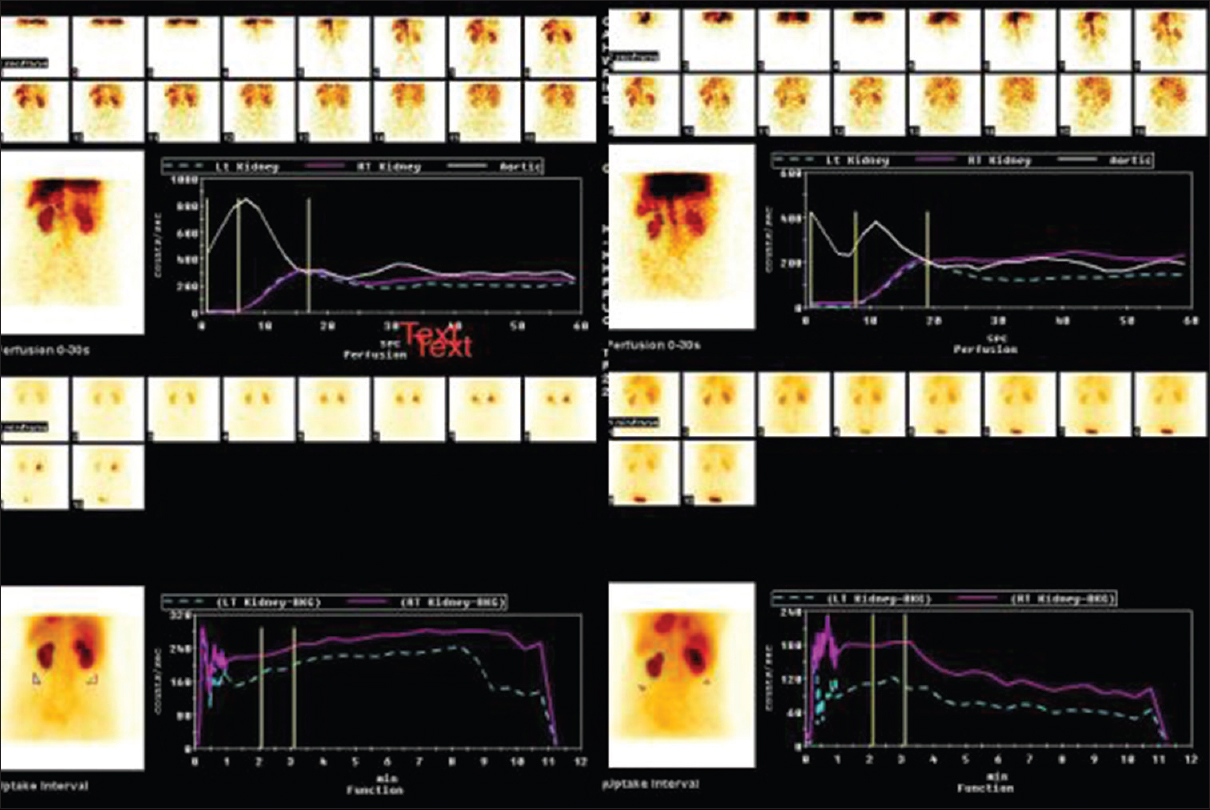
- Technetium Tc 99m-diethylenetriaminepentaacetic acid (Tc 99m-diethylene triamine pentaacetic acid) scan. Pre- and post-operative scan showing good perfusion and uptake by both kidneys (Gates method). In upper part of figure, white line shows aortic blood flow and violet and green (broken) lines shows right and left renal flow (perfusion) respectively. In lower part of figure violet and green (broken) lines shows right and left kidney excretion (function), respectively.
Statistical analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS Inc, Chicago, Illinois, USA) software version 17. Data were expressed in mean, median, and percentage. The normalcy of data was checked using One-Sample Kolmogorov–Smirnov Test. Statistical analysis was performed using Pearson Chi-square test, Student's t-test, Paired t-test, Fisher's exact test, one-way and two-way ANOVA, and multivariate linear regression model. Correlation was performed using Pearson coefficient of correlation. P = 0.05 or less was considered statistically significant.
RESULTS
A total of 52 patients were assessed at baseline and 3 months following surgery. Baseline and follow-up, DTPA scan could be performed in 45 patients because of logistic reasons. Demographic, clinical, pathological, and operative data are summarized in Table 1. Mean age at the time of presentation was 48.96 years and male preponderance was observed (71%). Majority of the tumors (75%) were incidentally detected. Fifteen (29%) patients had CrCl <60 ml/min/1.73 m2 at presentation. In 33 (64%), exophytic component of tumor was >50%, tumor located at upper, lower, and interpolar site in 48%, 37%, and 15%, respectively.
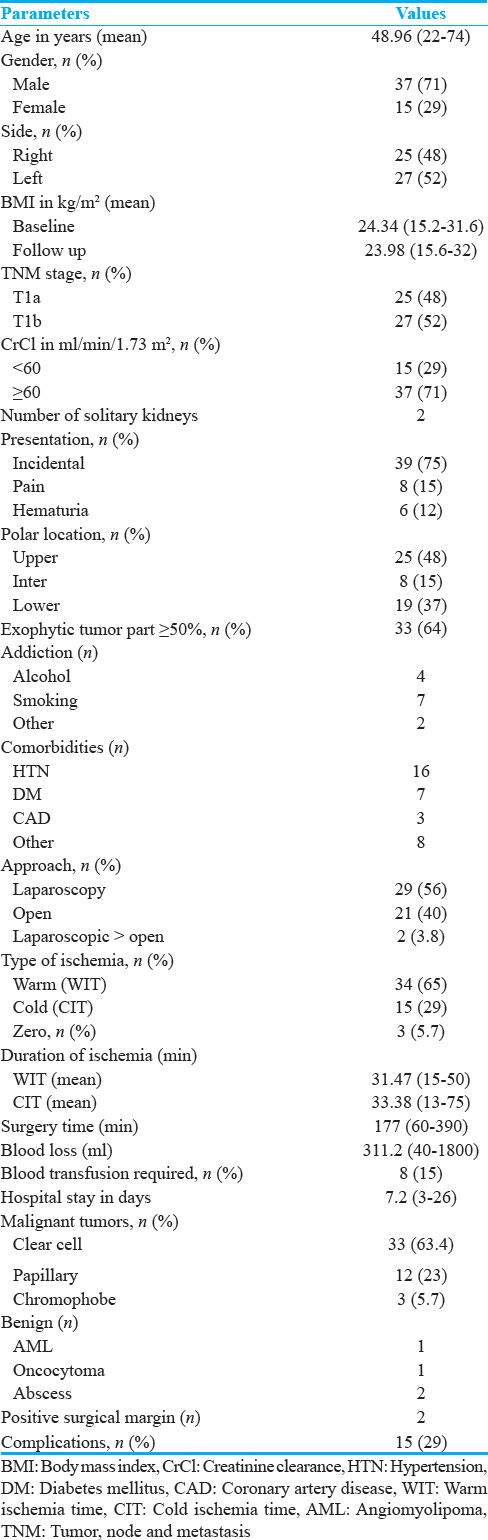
Majority of patients underwent laparoscopic partial nephrectomy, of these two patients required open conversion. The reason for conversion to open was excessive bleeding in one and unclear tumor demarcation in the other. The primary reasons for open surgery were surgeon's preference and hilar location of tumor. Three patients underwent NSS without clamping the vessels. Warm ischemia (only clamping the renal vessels without and surface cooling mechanisms) was created in 34 (65%) of patients during NSS. The time of vessel clamp was noted as warm ischemia time (WIT). Fifteen patients underwent both vessel clamping and surface cooling with ice slush. Cold ischemia time (CIT) was the total clamp time in these patients.
Mean duration of warm ischemia was 31.47 min (15–50) whereas mean cold ischemia duration was 33.38 min (13–75). Duration of warm ischemia was further categorized into two subgroups (>30 min in 14 patients and ≤30 min in 20 patients). Mean duration of surgery was 177 min (60–390) and it was more in laparoscopy cases. Mean blood loss was 311.2 ml (40–1800), in seven patients’ blood loss was ≥500 ml, and eight patients needed blood transfusion. Grade 1 and 2 Clavien-Dindo complications was noted in 12 (23%) and 8 (15.38%) patients, respectively.
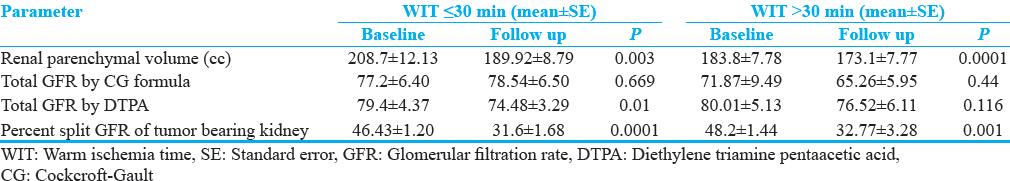
Quantitative and qualitative assessment of renal parenchyma in different group of ischemia is shown in Table 2. Baseline mean renal parenchymal volume of tumor-bearing kidney was 190cc (standard error [SE] ± 5.81). Following NSS, at 3 months’ follow-up, the renal volume reduced to 175.91cc (SE ± 4.73) which was statistically significant. The percentage split GFR of tumor-bearing kidney reduced significantly at follow-up from 51.36% (SE ± 2.11) to 31.8% (SE ± 1.32) whereas percent split GFR of opposite renal moiety showed a relative increase from 48.4% (SE ± 2.11) to 52.44% (SE ± 2.38). Change in renal parenchymal volume, total GFR by CG and DTPA, split GFR of tumor-bearing moiety was significant in all patients together and subgroups such as WIT, CIT, and zero ischemia time [Table 2]. When subgroups of WIT compared, change in renal parenchymal volume, total GFR by CG formula, split GFR of tumor-bearing, and opposite moiety was not different; however, total GFR by DTPA scan was significantly low in ≤30 WIT group [Table 3].
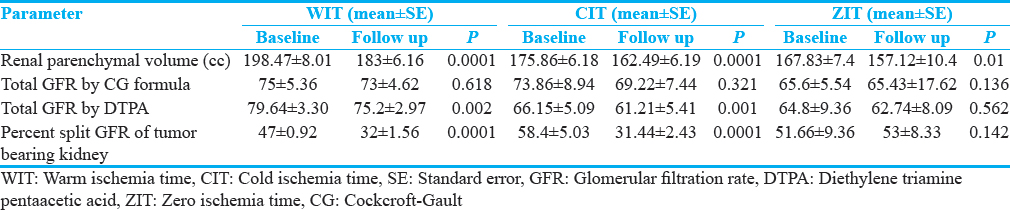
Preoperative CrCl <60 ml/min versus >60 ml/min, approach to surgery open versus laparoscopic and stage of tumor T1 versus T2 did not show any statistically significant differential effect in their change in renal parenchymal volume, total and percentage split GFR of tumor-bearing kidney.
On multivariate analysis
-
Age and hospital stay were independent variables for change in total GFR by DTPA scan (r = 0.609, P = 0.0001)
-
Approach (open/lap) of surgery was independent variable for decrease in split renal function of tumor-bearing kidney was associated with (r = 0.302, P = 0.04). Positive surgical margins were observed in two patients operated laparoscopically; both in follow-up imaging did not show any radiological evidence of tumor or recurrence till now.
DISCUSSION
Different authors have described multiple factors affecting renal function after NSS such as age, gender, tumor size, ischemia type and time, remaining renal volume, and postoperative complication. The individual contribution of each factor for final renal volume and function is still not very clear.[281011]
The upper limit of the ischemic time that affect renal function remains controversial to date. Based on the previous literature reports till 1980, 30 min is the generally accepted upper limit. Thompson et al., studied the effect of renal vessels clamping during partial nephrectomy in patients with a solitary kidney. They found that warm ischemic time >20 min and cold ischemic time >35 min was associated with a higher increase in serum creatinine levels. However many authors reported that renal parenchyma was independent of WIT and many factors play role.[12131415] Also review of the literature by Volpe et al., showed that even WIT >30 min have no adverse effects in patients with bilateral functioning kidneys,[16] in this study, WIT was >30 min in 14 patients. Nevertheless, ischemia should be kept as short as possible especially for patients with T1 or large tumor and patient with at risk of progression of renal functions.
Most surgeons prefer the use of vascular clamping to minimize blood loss, acquire good visibility of the tumor bed, allow safe assessment of oncologic margins, and enable quick hemostasis.[121317] This warm ischemia duration is proven to be an important modifiable factor, but still definitive upper limit is yet to be defined. It is well known that WIT leads to hypoxic-reperfusion injury to ipsilateral renal moiety which is directly related to the duration of ischemia, but the natural history of this damage is not well understood. Although every minute counts after the renal hilum is clamped, data suggest that safe WIT ranges between 20 and 25 min, but a limit of 30 min is considered safe time for WIT. Cold ischemia is well tolerated by kidney up to 120 min depending on technique used for cooling. Nevertheless, cold ischemia with ice slush should be kept as short as possible.[251819] In our study, CIT was in acceptable limit, whereas duration of warm ischemia exceeded 30 min in 14 patients. There was significant decrease in renal parenchymal volume, percentage split GFR, and total GFR by DTPA scan in both warm and cold ischemia group whereas GFR by CG formula was not changed. On subgroup comparison between WIT >30 min and <30 min, there was difference in percentage split renal function and loss of renal parenchyma only as shown in [Table 2]. In nonclamp group, only renal parenchymal loss was significant and rest of the parameters did not show any significant change. The magnitude of the decrease in GFR appears to be influenced by three factors – (1) functional volume loss, (2) ischemia-related acute kidney injury, and (3) mechanical trauma effects. In review of literature, Volpe mentioned that complex and large tumor need longer ischemia and probably large nondiseased parenchyma loss and eventually more loss of renal function. Furthermore, surgical technique plays an important role in the form of renorrhaphy; intraoperative ultrasound helps in identifying true margins and also avoid fear of cutting into tumor.[1916] In this study, the surgical steps were almost same for all surgeons, but renorrhaphy was probably bit different for the case to case. We also have dedicated intraoperative ultrasound probe to guide surgical steps whenever necessary.
Serum creatinine level is an effective parameter for monitoring renal functional changes in patients with a solitary kidney; it is not an effective parameter for cases with a normal opposite kidney. In the study by Joniau et al., T1a and T1b were compared and found that patient characteristics were different; however, the overall complication rate, pathological, and oncological variables were not significantly different. Many investigators have suggested that it is the inherent tumor biology rather than surgical approach or tumor size that has the major influence on outcomes after NSS for T1 RCC.[1520] In this study also, there was no homogenous change in GFR by DTPA and CG formula, which is based on serum creatinine.
It is assumed that function of the normal side did not change. However, functional adaptation occurs in the contralateral side, and to precisely evaluate any damage caused by NSS, the quantitative split renal function should be investigated.[13] Data have shown that the percentage decrease in functional kidney volume correlates strongly with the percentage decrease in GFR at late points after NSS. These data also demonstrate that volume loss impacts GFR to a greater degree than ischemic injury when ischemia time is maintained within acceptable time limits.[2122] The most important variables associated with short- and long-term renal function after NSS are the quality and the quantity of kidney remaining after the procedure. In our study, also there is a significant renal volume loss, but it is not reflected by a similar decrease in GFR both by DTPA and CG formula which could possibly be due to transient increase in percentage renal function of opposite moiety. Although the quantity preserved has traditionally been considered a nonmodifiable factor, there are some authors who have highlighted the potential importance of the precision of excision and reconstruction in an effort to optimize this parameter.[20]
Renal scintigraphy is the only method that allows clinicians to quantify real changes in split renal-functional loss of the tumor-bearing kidney after NSS.[818] In our study, we used DTPA renal scan to evaluate split GFR; it could be done in 45 patients and Gates method was used instead of Plasma method. Lane et al., studied 1169 patients retrospectively and found that increasing age, male gender, and larger tumor size were significant predictors of fall in postoperative GFR. Maurice et al., studied various factors affecting postsurgery renal volume loss and found that male sex, larger tumors, endophytic tumors, open approach, increased bleeding, and higher surgeon volume were independently associated with more volume loss[2323] However, in our study, age, gender, duration of ischemia, exophytic component of tumor, and approach for surgery were independent variable for renal parenchymal volume, total and split GFR, whereas size, stage, polar location of tumor, duration of surgery, preoperative CKD, and need of blood transfusion did not affect change in renal volume and function in the follow-up period. According to literature patients with preoperatively compromised renal function are prone for decreased renal function and also the duration of ischemia should strictly keep as short as possible. In this study, 29% patient's GFR was <60 ml/min, preoperatively, however, there was no deterioration of renal function in postoperatively; probably, we did not include advanced CKD patients. Approach to surgery has mixed results in term of postoperative outcome; one study showed better postoperative renal function after laparoscopic approach while other showed no difference, in this study, there was no effect of the approach of surgery.[162425]
CONCLUSION
In this prospective study, we studied that quality and quantity of renal tissue that is preserved after surgery have effect on short-term renal function. Functional renal parenchymal volume correlates to split GFR of ipsilateral moiety, and functional parenchyma further depends on duration of ischemia not the type of ischemia. Duration of ischemia is modifiable factor; it should be kept short for both warm as well as cold. Further studies are needed with measurement of tumor complexity to study true loss of ipsilateral renal function.
Following are shortcoming of the present study.
-
Small sample size
-
Surgery was not performed by a single surgeon
-
Surgical technique could have been more elaborative for different complexity of tumor
-
Intraoperative cut specimen could have been seen to look loss of normal renal parenchyma
-
Renal nephrometry score is standard tool to calculate complexity of tumor; we did not consider all parameter of nephrometry score
-
GFR by DTPA scan could not be performed in all patients
-
GFR by DTPA camera method is not as accurate as DTPA by Plasma method.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Mr. Tikam Dadhich for his valuable support.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/15/230189.
REFERENCES
- Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363-8.
- [Google Scholar]
- Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011;186:405-10.
- [Google Scholar]
- The expanding role of partial nephrectomy: A critical analysis of indications, results, and complications. Eur Urol. 2010;57:214-22.
- [Google Scholar]
- Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181-5.
- [Google Scholar]
- Correlation between differential renal function estimation using CT-based functional renal parenchymal volume and (99m) Tc – DTPA renal scan. Indian J Urol. 2012;28:414-7.
- [Google Scholar]
- Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. 2012;188:51-7.
- [Google Scholar]
- Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468-71.
- [Google Scholar]
- Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci (Lond). 1984;66:613-9.
- [Google Scholar]
- Factors influencing renal function reduction after partial nephrectomy. J Urol. 2009;181:48-53.
- [Google Scholar]
- Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012;79:356-60.
- [Google Scholar]
- Comparison of costs and complications of radical and partial nephrectomy for treatment of localized renal cell carcinoma. Urology. 2002;59:211-5.
- [Google Scholar]
- Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006;7:735-40.
- [Google Scholar]
- National trends in the use of partial nephrectomy: A rising tide that has not lifted all boats. J Urol. 2012;187:816-21.
- [Google Scholar]
- Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol. 2009;56:625-34.
- [Google Scholar]
- Predictors of precision of excision and reconstruction in partial nephrectomy. J Urol. 2014;192:30-5.
- [Google Scholar]
- The impact of ischemia time during open nephron sparing surgery on solitary kidneys: A multi-institutional study. J Urol. 2007;177:471-6.
- [Google Scholar]
- Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur Urol. 2009;55:209-15.
- [Google Scholar]
- Correlation between loss of renal function and loss of renal volume after partial nephrectomy for tumor in a solitary kidney. J Urol. 2008;179:1284-8.
- [Google Scholar]
- Parenchymal volume preservation and ischemia during partial nephrectomy: Functional and volumetric analysis. Urology. 2013;82:263-8.
- [Google Scholar]
- Outcome of nephron-sparing surgery for T1b renal cell carcinoma. BJU Int. 2009;103:1344-8.
- [Google Scholar]
- Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol. 2011;185:421-7.
- [Google Scholar]
- Renal ischemia and function after partial nephrectomy: A Collaborative review of the literature. Eur Urol. 2015;68:61-74.
- [Google Scholar]
- Predictors of excisional volume loss in partial nephrectomy: Is there still room for improvement? Eur Urol. 2016;70:413-5.
- [Google Scholar]
- Recovery of renal function after open and laparoscopic partial nephrectomy. Eur Urol. 2010;58:596-601.
- [Google Scholar]






