Translate this page into:
Evaluation of Orthopedic Metal Artifact Reduction Application in Three-Dimensional Computed Tomography Reconstruction of Spinal Instrumentation: A Single Saudi Center Experience
Address for correspondence: Dr. Amir Monir Ali, Department of Radiodiagnosis, Faculty of Medicine, Mansoura University, Egypt. Department of Medical Imaging, Sultan Bin Abdulaziz Humanitarian City, Riyadh 13571-6262, Kingdom of Saudi Arabia. E-mail: amirmonirali@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim of the Study:
The aim of the study was to evaluate the commercially available orthopedic metal artifact reduction (OMAR) technique in postoperative three-dimensional computed tomography (3DCT) reconstruction studies after spinal instrumentation and to investigate its clinical application.
Materials and Methods:
One hundred and twenty (120) patients with spinal metallic implants were included in the study. All had 3DCT reconstruction examinations using the OMAR software after obtaining the informed consents and approval of the Institution Ethical Committee. The degree of the artifacts, the related muscular density, the clearness of intermuscular fat planes, and definition of the adjacent vertebrae were qualitatively evaluated. The diagnostic satisfaction and quality of the 3D reconstruction images were thoroughly assessed.
Results:
The majority (96.7%) of 3DCT reconstruction images performed were considered satisfactory to excellent for diagnosis. Only 3.3% of the reconstructed images had rendered unacceptable diagnostic quality.
Conclusion:
OMAR can effectively reduce metallic artifacts in patients with spinal instrumentation with highly diagnostic 3DCT reconstruction images.
Keywords
Orthopedic metal artifact reduction
spinal instrumentation
three-dimensional computed tomography

INTRODUCTION
Spinal metallic implants are widely used in modern spinal surgery for increasing incidence and expanding etiologies. Magnetic resonance imaging evaluation is limited due to the magnetic severe susceptibility artifacts; so, computed tomography (CT) is now the method of choice for the postoperative assessment of spinal metallic instrumentation.[12] However, the presence of spinal metal implants produces considerable artifacts on CT images, resulting in marked degradation of image quality and subsequent changes of CT attenuation values of the surrounding tissues, which seriously influences the reliability of the postoperative three-dimensional CT (3DCT) assessment of spinal instrumentation.[3] Hence, many studies investigated the metallic artifact reducing methods used in clinical practice including linear interpolation,[4] spline interpolation,[5] simultaneous algebraic reconstruction,[6] and simultaneous iterative reconstruction[7] techniques.
Orthopedic metal artifact reduction algorithm (OMAR) software is a commercial algorithm produced by Philips Health System, Cleveland, USA, that is used to remove metal artifacts by acquisition data alteration. Much iteration makes the new technique suitable for internal fixation of extended metal implants such as spinal plates, rods, and screws.[8] However, there are scarce reports regarding the evaluation of OMAR in postoperative 3DCT scans of spinal instrumentation.
The aim of this qualitative study was to assess the feasibility of the use of OMAR in the postoperative 3DCT reconstruction of spinal implants and investigate its clinical application.
MATERIALS AND METHODS
This study was conducted in Sultan Bin Abdulaziz Humanitarian City (SBAHC), Riyadh, Kingdom of Saudi Arabia. One hundred and twenty patients with spinal metallic instrumentation were included in this study from June to December 2017; 55 new cases (45.8%) and 65 cases coming for regular follow-up (54.2%). The segmental spinal regions were 9 cervical (7.5%), 101 dorsolumbar (84.2%), and 10 lumbar (8.3%). The study population included 108 (90%) males, with a median age of 31 and 12 (10%) females with the median age of 27. Etiology for spinal instrumentation included 49 (40.8%) posttraumatic fractures cases, 68 (56.7%) postscoliosis correction, and 3 (2.5%) post-tuberculosis (TB) cases. Informed consents and approvals of the SBAHC Scientific Research Ethical Committee were obtained for all patients before the CT examinations.
CT scans with 3D reconstruction were performed on a 64-slice CT scanner (Ingenuity; Philips Medical Systems, Cleveland, USA). Parameters used were helical CT protocol, iterative reconstruction: IDose Level 3 (range, 1–5), slice thickness 0.5 mm, slice interval 0.5 mm, matrix 512 × 512, collimation 64 × 0.625 mm, OMAR software, bone window (YD kernel, level 800 HU, width 2000 HU), and soft window (Soft kernel B). The degree of metallic artifacts and clarity of the scanned vertebrae of the 3D reconstruction images using OMAR were evaluated to determine their diagnostic acceptance.
Image quality evaluation was conducted on the source bone and soft-tissue windows as well as the 3D reconstruction images. A blinded consultant radiologist, with more than 15 years of experience in radiological diagnosis, conducted the subjective evaluations including severity of the spinal metallic artifacts, density of the related paraspinal muscles, clarity of the intermuscular fat planes, and definition of the scanned vertebral elements using a 5-point scale [Table 1].[1] Images scoring 3 or more were considered radiologically satisfactory.
RESULTS
In the produced spinal 3D-reconstructed images after OMAR application, the severity of the fixing metallic artifacts was significantly reduced, paraspinal muscles were more homogeneous in density, the intermuscular fat planes and planes between bone and instrumentation were clearer.
Images of 116 (96.7%), out of the 120 cases included in this study, were considered radiologically satisfactory to excellent. The score range of severity of instrumentation artifacts was 3–4 points, with the median of 4 points; and the range of definition of vertebrae was 3–5 points, with the median of 4 points. Thirty-six cases (30%) took the highest score of 5, 51 cases (42.5%) got Score 4, and 29 (24.2%) got Score 3. The four unacceptable cases (3.3%) were scored only one point for the ill-definition of the related vertebral bodies and posterior neural arches (2 cases) and inability to determine the intraspinal extension of TB-associated perispinal soft-tissue mass component (2 cases).
DISCUSSION
Artifacts caused by the presence of metallic implants in the CT scanned volume, such as spine, hip prostheses, or dental fillings, appear as dark and bright streaks across the reconstructed image.[9] Metallic artifacts can severely degrade the image quality and hence limit the diagnostic value of a CT scan.[10]
Metallic artifacts in CT scans are produced by beam hardening and photon starvation.[11] Photon starvation artifacts occur when the beam traverse materials with high-attenuation coefficients, which leads to a small amount of photons reaching the machine detectors and results in markedly noisy images.[12] The noise is multiplied in the reconstruction images, and the resulting streaks can be seen clearly. Beam hardening means that low energy photons are attenuated more than high energy photons when passing through the scanned object.[13] This effect is more prominent when the beam passes through very high-density materials such as metallic implants.[10]
Many solutions have been used to reduce metal artifacts; these are most often insufficient in terms of image quality for diagnosis. One approach is to increase the tube peak voltage; however, this may only reduce metallic artifact to a minor degree but may not improve imaging of larger implants. Increasing tube current, on the other hand, would lead to a higher radiation dose to the patient and may not have a considerable impact on image quality either.[11] Other solutions such as gantry tilting, or using lower attenuating materials in implants, may only be possible in specific situations and are effective to a minor extent. Multiple metallic artifact reduction algorithms working on raw projection data, such as modified iterative reconstruction and projection interpolation techniques, have been shown to be a more general and effective technique for reducing artifacts.[1213] Kidoh et al.[14] confirmed the value of OMAR with dental implants. Jeong et al.[15] discussed the value of the technique in improving the diagnostic accuracy of abdominal CT studies with metal artifacts. Li et al.[16] concluded that the algorithm could improve the quality of CT images taken for radiation therapy planning. Hu et al. conducted a study on a small scale of patients with the same conclusion.[1]
The OMAR algorithm used with Philips CT has previously been shown to improve CT number accuracy in images of orthopedic implants.[17] OMAR is an iterative projection modification method optimized for imaging orthopedic implants. The data acquired with the scanner are used as input into an iterative loop, where the output is a correction image that is subtracted from the input image. OMAR uses segmentation and replaces data points identified as metal with interpolated values. When using the OMAR technique, an uncorrected data volume is always automatically reconstructed.[12] In this study, we evaluated the use of this algorithm in postoperative 3D scans for spinal instrumentation and investigated its clinical application.
For elimination of metallic artifacts, the source data of the primary images, the sole implant images, and the scanned tissue images were compared and computed continuously until no metal data were detected, and the final images were reconstructed by filtered back projection technique.[18] Based on these calculations, metal artifacts are eliminated gradually; hence, OMAR is beneficial for sizable metallic implants. To increase the accuracy of metal artifact reduction, OMAR generates specific images for the metal based on CT attenuation on the original image, with a value of 0 for the metal density and 1 for the nonmetal densities; thus, the data acquisition is only for the metal domain with a value of 0. OMAR is supposed to have no side effects on the nonmetal parts of the images. After removal of the metal domain from the original acquired data, interpolation is applied to fill the lost data to reduce the variation in the produced images.[19]
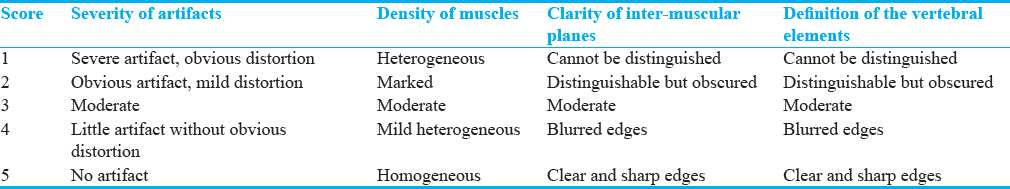
The clinical application of OMAR for postspinal instrumentation was performed on 120 patient population coming for regular postoperative evaluations in SBAHC, one of the most prestigious rehabilitation centers in the Kingdom of Saudi Arabia, with two-international accreditations of CARF and JCI and one national accreditation of CBAHI. Cases included spinal instrumentation for posttraumatic [Figure 1] (49 cases), post-TB [Figure 2] (3 cases), postscoliosis correction [Figure 3] (68 cases) etiologies. Figure 4 represents OMAR score 5, while figures 5–8 represent lower OMAR scores. The results indicated that the images of 116 cases (96.7%) were scored 3 or more and were accepted by the radiologist, which confirmed the feasibility of OMAR in spinal instrumentation imaging. The degree of metallic artifacts and the definition of the adjacent vertebrae were all evaluated 4 points, impressive of little metallic artifacts with no obvious distortion. With the resulting clear 3D spinal reconstructions, the spinal surgeons could be sure about the relationship between vertebrae and the fixing hardware (e.g., the proper placing and depth of transpedicular screws, no injury of the related nerve roots, and no loosening or malplacement), evaluate the status of vertebrae surrounding the implant for osteoporosis or infection, and follow-up the healing process of fractures. Thus, this technique would help accurate postoperative assessment of spinal fixation of different reasons.[20] The 3D-reconstructed images were unsatisfactory in only four patients (3.3%) in this study; this could be attributed to the metal being too near to the surface [Figure 2] (one case) or too extensive (two cases of both anterior and posterior fixation and one case of posterior and lateral fixation), which can lead to faulty reconstruction by OMAR.
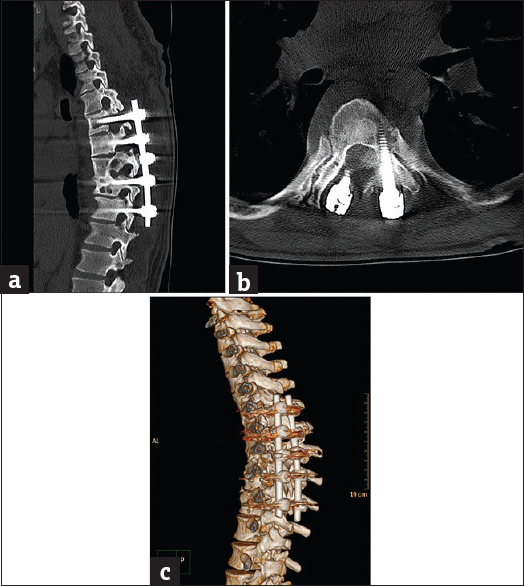
- Case 1: A 42-year-old male posttraumatic vertebral fracture patient with complicated spinal instrumentation and extensive vertebral osteolysis. (a) Sagittal bone window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm showing the metallic implants with a clear definition of the vertebral details and minimal artifact streaks (Score 4). (b) Three dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing the fixing metallic rods with minimal intervening streak artifacts. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, right posterior-lateral projection showing the extensive vertebral osteolysis with superior bone details and minimal streak artifacts.
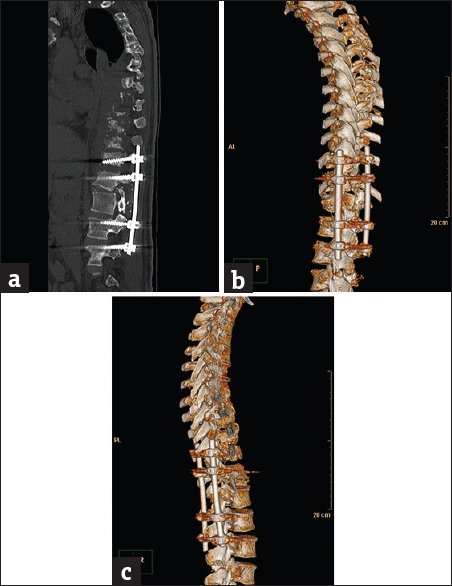
- Case 2: A 66-year-old male patient with spinal instrumentation for tuberculosis. (a) Sagittal soft-tissue window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm showing severe image degradation with obscuring artifact streaks (Score 1). (b) Axial bone window using orthopedic metal artifact reduction algorithm showing the metallic implants with marked veiling artifacts, ill-definition of the vertebral details and failure to delineate the extent of the perispinal tuberculosis lesion. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing marked haziness of the bony details with marked streak artifacts.
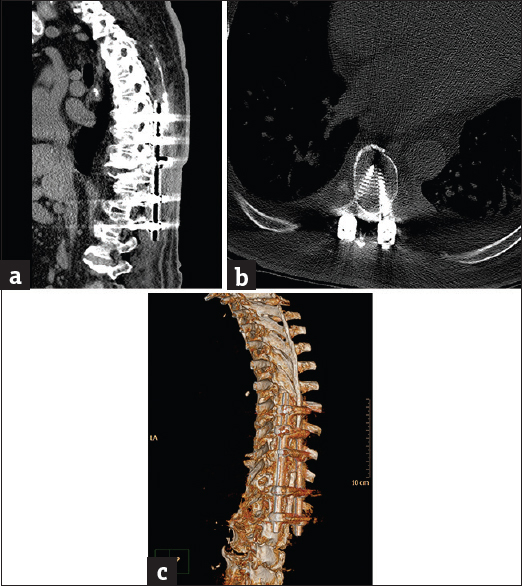
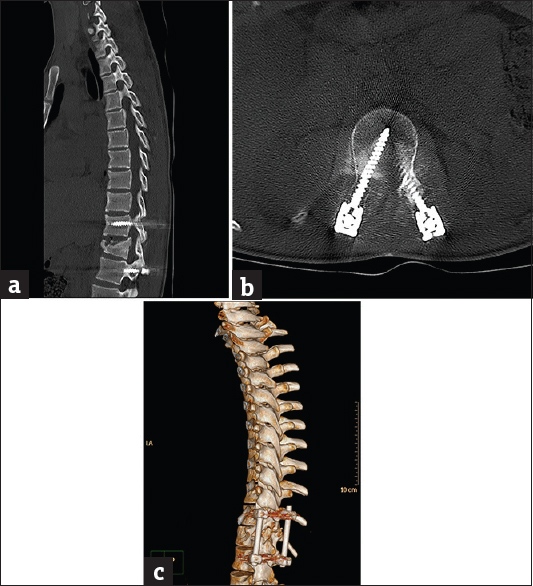
- Case 3: A 27-yar-old female patient with corrective spinal instrumentation for kyphoscoliotic deformity. (a) Sagittal bone window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm showing excellent vertebral details with almost absence of significant obscuring artifacts (Score 5). (b) Axial bone window using orthopedic metal artifact reduction algorithm, showing the metallic implants with clear vertebral and paraspinal muscle details. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing the whole length of the corrective implant with clear anatomical relations.
- Case 4: A 33-year-old patient with post-tuberculosis spinal fixation. (a) Sagittal bone window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm showing excellent vertebral details with almost absence of significant obscuring artifacts (Score 5). (b) Axial bone window using orthopedic metal artifact reduction algorithm, showing the tuberculous perispinal mass with clear intraspinal extension. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing the fixing implant with well-defined tuberculosis bone destruction.
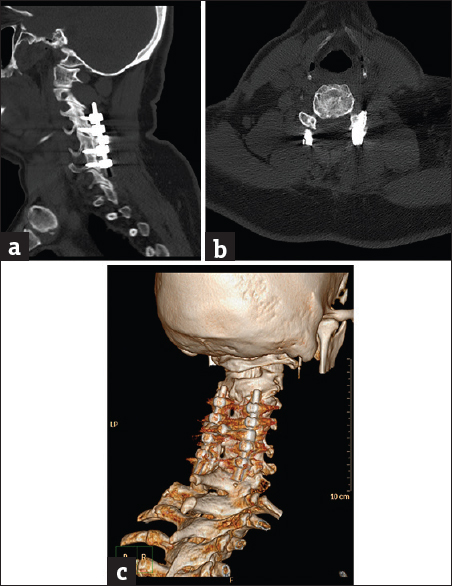
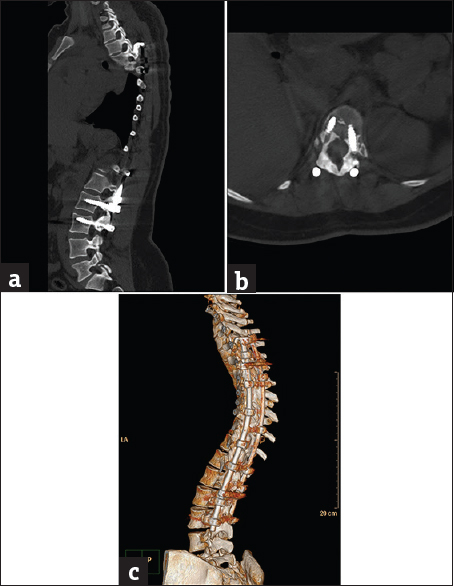
- Case 5: A 36-year-old male patient with posttraumatic cervical spine fixation. (a) Sagittal bone window reformat of the cervical spine using orthopedic metal artifact reduction algorithm, showing internal fixation through a posterior approach with good vertebral details (Score 4). (b) Axial bone window using orthopedic metal artifact reduction algorithm, showing the good healing process of the vertebral fracture with no significant veiling artifacts. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, right posterior-lateral projection showing the cervical instrumentation with satisfactory details.
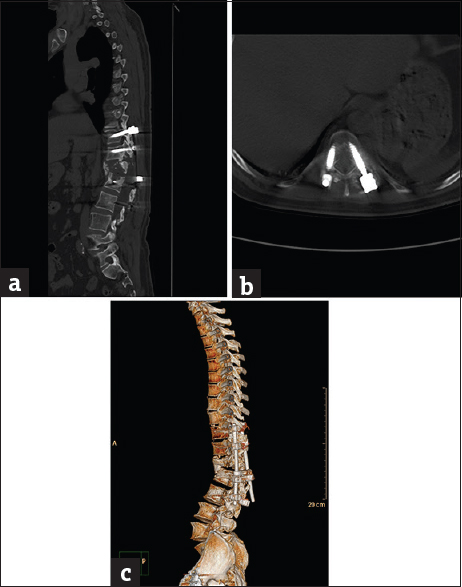
- Case 6: A 22-year-old male patient with post-L1 spinal fracture fixation. (a) Sagittal bone window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm, showing marked posterior cortical displacement of the fractured vertebra encroaching upon the spinal canal with satisfactory bone details (Score 3). (b) Axial bone window using orthopedic metal artifact reduction algorithm, showing satisfactory bone details yet, obscured adjacent paraspinal muscles. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing the spinal canal encroachment with satisfactory details.
- Case 7: A 55-year-old male patient with postfixation infection and hardware failure. (a) Sagittal bone window reformat of the dorsolumbar spine using orthopedic metal artifact reduction algorithm, showing marked loosening of the lower fixing pedicular screws with evident displacement and extensive osteolysis (Score 4). (b) Axial bone window using orthopedic metal artifact reduction algorithm showing the relation between the detached screws and the related vertebra. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, left posterior-lateral projection showing the evident hardware failure and vertebral destruction.
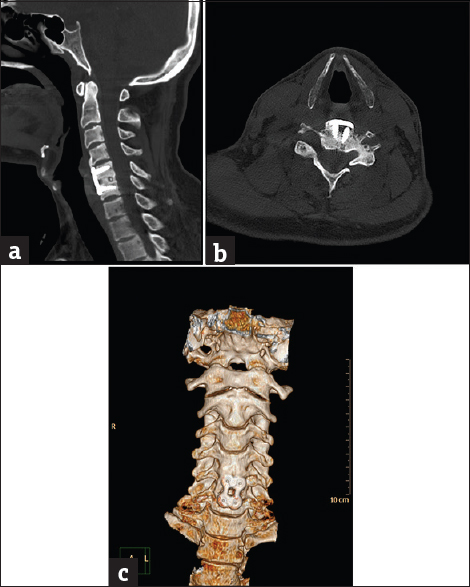
- Case 8: A 26-year-old female patient with nonunited vertebral fracture. (a) Sagittal bone window reformat of the cervical spine using orthopedic metal artifact reduction algorithm showing very clear details of the anterior spinal fixation (Score 5). (b) Axial bone window using orthopedic metal artifact reduction algorithm showing the gapping nonunited fractured posterior neural arch with the almost total absence of veiling artifacts. (c) Three-dimensional computed tomography color-coded reconstruction with orthopedic metal artifact reduction, anterior projection showing the good cervical alignment despite the nonunited fracture.
Philips recommends that this algorithm is not suitable for imaging of stents, external fixation metals, implanted devices near skin surface, metals near air pockets, and surgical screws or clips. There are some cases where OMAR should be avoided as outlined in the contraindications section.[2122] These commonly occur when the metal is in close proximity to air or lung tissue or small metal object (e.g. stents) within iodinated contrast. The radiologist should be encouraged to cross reference the uncorrected images with the OMAR dataset when there are unsatisfactory construction images. Since the system will always reconstruct both sets of images whenever OMAR is selected, the uncorrected images are readily available.[23]
To have a complete evaluation of this OMAR technique, it would be of interest to perform a quantitative image analysis of CT numbers as a complement to the qualitative analysis carried out in this study. Other visual grading studies of specific spinal regions, cervical, dorsal or lumbosacral images, or based on the etiology for spinal instrumentation, would be complementary to this study.
CONCLUSION
From this postspinal instrumentation study on 120 patients, it was concluded that, using the OMAR algorithm, combined with 3D reconstruction, much improved the produced images in terms of image quality, diagnostic accuracy, and better anatomical structure visualization, avoiding subjective density overrides of artifact regions on uncorrected images. Further quantitative image analysis studies for OMAR evaluation would be of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/11/227125.
REFERENCES
- Value and clinical application of orthopedic metal artifact reduction algorithm in CT scans after orthopedic metal implantation. Korean J Radiol. 2017;18:526-35.
- [Google Scholar]
- Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol. 2002;6:5-17.
- [Google Scholar]
- Postoperative evaluation after anterior cruciate ligament reconstruction: Measurements and abnormalities on radiographic and CT imaging. Korean J Radiol. 2016;17:919-30.
- [Google Scholar]
- Computed tomographic metal artifact reduction for the detection and quantitation of small features near large metallic implants: A comparison of published methods. J Comput Assist Tomogr. 2008;32:621-9.
- [Google Scholar]
- A virtual sinogram method to reduce dental metallic implant artefacts in computed tomography-based attenuation correction for PET. Nucl Med Commun. 2010;31:22-31.
- [Google Scholar]
- Simultaneous algebraic reconstruction technique (SART): A superior implementation of the art algorithm. Ultrason Imaging. 1984;6:81-94.
- [Google Scholar]
- Iterative methods for the three-dimensional reconstruction of an object from projections. J Theor Biol. 1972;36:105-17.
- [Google Scholar]
- Metal Artifact Reduction for Orthopedic Implants (O-MAR) Available from: http://www.clinical.netforum.healthcare.philips.com/us_en/Explore/White-Papers/CT/Metal-Artifact-Reduction-for-Orthopedic-Implants- (O-MAR)#
- [Google Scholar]
- Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. Radiographics. 2007;27:791-803.
- [Google Scholar]
- Clinical evaluation of a newly developed method for avoiding artifacts caused by dental fillings on X-ray CT. Radiol Phys Technol. 2008;1:115-22.
- [Google Scholar]
- An adaptive approach to metal artifact reduction in helical computed tomography for radiation therapy treatment planning: Experimental and clinical studies. Int J Radiat Oncol Biol Phys. 2005;62:1224-31.
- [Google Scholar]
- Reduction of dental metallic artefacts in CT: Value of a newly developed algorithm for metal artefact reduction (O-MAR) Clin Radiol. 2014;69:e11-6.
- [Google Scholar]
- Usefulness of a metal artifact reduction algorithm for orthopedic implants in abdominal CT: Phantom and clinical study results. AJR Am J Roentgenol. 2015;204:307-17.
- [Google Scholar]
- Clinical evaluation of a commercial orthopedic metal artifact reduction tool for CT simulations in radiation therapy. Med Phys. 2012;39:7507-17.
- [Google Scholar]
- The CT number accuracy of a novel commercial metal artifact reduction algorithm for large orthopedic implants. J Appl Clin Med Phys. 2014;15:4597.
- [Google Scholar]
- Metal artefact reduction in CT imaging of hip prostheses – An evaluation of commercial techniques provided by four vendors. Br J Radiol. 2015;88:20140473.
- [Google Scholar]
- Virtual monochromatic spectral imaging with fast kilovoltage switching: Reduction of metal artifacts at CT. Radiographics. 2013;33:573-83.
- [Google Scholar]
- Spectral CT with metal artifacts reduction software for improvement of tumor visibility in the vicinity of gold fiducial markers. Radiology. 2012;263:696-705.
- [Google Scholar]
- Normalized metal artifact reduction (NMAR) in computed tomography. Med Phys. 2010;37:5482-93.
- [Google Scholar]
- Fast iterative algorithm for metal artifact reduction in X-ray CT. Acad Radiol. 2000;7:607-14.
- [Google Scholar]
- An evaluation of three commercially available metal artifact reduction methods for CT imaging. Phys Med Biol. 2015;60:1047-67.
- [Google Scholar]






