Translate this page into:
Transjugular Intrahepatic Portosystemic Shunt Dysfunction: Concordance of Clinical Findings, Doppler Ultrasound Examination, and Shunt Venography
Address for correspondence: Dr. Ron Charles Gaba, Department of Radiology, Division of Interventional Radiology, University of Illinois Hospital and Health Sciences System, 1740 West Taylor Street, MC 931, Chicago, 60612 IL, USA. E-mail: rgaba@uic.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
The objective of this study was to evaluate the concordance between clinical symptoms, Doppler ultrasound (US), and shunt venography for the detection of stent-graft transjugular intrahepatic portosystemic shunt (TIPS) dysfunction.
Materials and Methods:
Forty-one patients (M:F 30:11, median age 55 years) who underwent contemporaneous clinical exam, Doppler US, and TIPS venography between 2003 and 2014 were retrospectively studied. Clinical symptoms (recurrent ascites or variceal bleeding) were dichotomously classified as present/absent, and US and TIPS venograms were categorized in a binary fashion as normal/abnormal. US abnormalities included high/low (>190 or <90 cm/s) TIPS velocity, significant velocity rise/fall (>50 cm/s), absent flow, and return of antegrade intra-hepatic portal flow. Venographic abnormalities included shunt stenosis/occlusion and/or pressure gradient elevation. Clinical and imaging concordance rates were calculated.
Results:
Fifty-two corresponding US examinations and venograms were assessed. The median time between studies was 3 days. Forty of 52 (77%) patients were symptomatic, 33/52 (64%) US examinations were abnormal, and 20/52 (38%) TIPS venograms were abnormal. Concordance between clinical symptoms and TIPS venography was 48% (25/52), while the agreement between US and shunt venography was 65% (34/52). Clinical symptoms and the US concurred in 60% (31/52) of the patients. The sensitivity of clinical symptoms and US for the detection of venographically abnormal shunts was 80% (16/20) and 85% (17/20), respectively. Both clinical symptoms and the US had low specificity (25%, 8/32 and 50%, 16/32) for venographically abnormal shunts.
Conclusion:
Clinical findings and the US had low concordance rates with TIPS venography, with acceptable sensitivity but poor specificity. These findings suggest the need for improved noninvasive imaging methods for stent-graft TIPS surveillance.
Keywords
Concordance
Doppler ultrasound
dysfunction
surveillance
transjugular intrahepatic portosystemic shunt

INTRODUCTION
The use of expanded polytetrafluoroethylene (e-PTFE) stent-grafts for transjugular intrahepatic portosystemic shunt (TIPS) creation has dramatically decreased the occurrence of shunt dysfunction as compared to bare metal stent predecessors. The current stent-graft TIPS affords primary patency rates approximating 80–90% at 1-year,[1] in contrast to patency rates below 50% at 1 year for bare metal stent TIPS.[2] Historically, the high frequency of bare metal stent shunt dysfunction led to the adoption of Doppler ultrasound (US) surveillance to detect shunt dysfunction prior to the onset of clinical symptoms,[34] and this practice has largely translated into clinical practice for contemporary TIPS.[5] Yet, it remains unclear if US protocols and criteria designed for the detection of bare metal stent TIPS dysfunction are applicable to stent-grafts.[678] As stent-grafts are increasingly utilized, they currently account for approximately 80% of stents used in modern TIPS procedures[9] – patient follow-up practices should ideally be based on evidence derived from the investigation of this device rather than older models. To this end, the high primary patency rates following stent-graft TIPS creation demand re-evaluation of the clinical utility of their routine surveillance with Doppler US. Therefore, this study aimed to evaluate the concordance between clinical symptoms, Doppler US examination, and shunt venography for the detection of TIPS dysfunction in the e-PTFE stent-graft era.
MATERIALS AND METHODS
Study design, clinical setting, and patients
This single-center, Institutional Review Board-approved retrospective study culled patients from a registry of 234 patients who underwent 236 technically successful TIPS procedures at a tertiary care, university-affiliated hospital, between 2003 and 2014. From these cases, procedures utilizing bare-metal stents for TIPS creation (n = 9) were excluded, rendering 227 patients who underwent stent-graft TIPS creation. Among these patients, 74 individuals underwent 91 TIPS venography studies for possible shunt revision during the study period of January 2003 to December 2015. Of those patients who underwent venography, 33 persons with 39 corresponding venography procedures were excluded due to lack of paired US examinations preceding intervention. The remaining 41 patients who underwent 52 TIPS venography studies thus comprised the final study cohort. Patient demographics, liver disease data, and pertinent clinical presentation information are shown in Table 1.

Transjugular intrahepatic portosystemic shunt procedures
The technique for TIPS creation has been previously described.[1011] Procedures were performed in the interventional radiology (IR) suite under general anesthesia. The right jugular venous access was obtained with the insertion of a 10 Fr sheath followed by the right atrial pressure measurement. The right or middle hepatic vein selection preceded the performance of free and wedged hepatic venography. A Rösch-Uchida transjugular liver access set (Cook Medical Co., Bloomington, IN, USA) was then used to access the right or left portal vein. After catheter introduction, the portal pressure was measured, portal venography was performed, and the liver tract was dilated. Next, 10 mm VIATORR® TIPS endoprostheses (W.L. Gore and Associated, Flagstaff, AZ, USA) were deployed followed by balloon dilation. Final portal and right atrial pressures were measured, followed by shunt venography. Occlusion of gastroesophageal varices, usually with metallic coils or vascular plugs, was performed at the discretion of the primary IR operator.
Postprocedure care, clinical follow-up, and Doppler ultrasound surveillance
Patients were monitored in an Intensive Care Unit after TIPS creation. Immediate postprocedure follow-up was performed while patients were still hospitalized. Future follow-up appointments occurred in an outpatient hepatology clinic. Return of clinical symptoms was defined by recurrent or persistent ascites or hydrothorax or recurrent variceal bleeding after TIPS creation.
After hospital discharge, US surveillance of TIPS was prescribed. Shunts were routinely evaluated sonographically at 1-week, 1-month, 3-month, 6-month, and 1-year post-TIPS, with annual surveillance thereafter. US examinations were performed by the American Registry for Diagnostic Medical Sonography-certified US technicians with more than 10 years of clinical experience using high-resolution scanners (Logiq e9; GE Healthcare, Little Chalfont, United Kingdom). Grayscale and color Doppler images with spectral waveforms were obtained. The TIPS performance parameters evaluated at each Doppler US examination included shunt patency and peak shunt velocity at the portal venous end, mid-shunt, and hepatic venous end. Shunt velocities–recorded in cm/s–were typically measured in suspended respiration (if possible) and using angle correction. The comparison was made for interval changes in TIPS peak shunt velocity since the prior scan. Other recorded data included main portal vein velocity and directionality of flow in the intrahepatic portal veins.
Gray-scale and Doppler US shunt abnormalities were diagnosed using published criteria,[12] and consisted of high (>190 cm/s) or low (<90 cm/s) TIPS velocity, significant temporal alteration in TIPS velocity (>50 cm/s) since the prior Doppler US examination (when available), absent flow within the shunt, and return of antegrade intra-hepatic portal flow. Data on portal vein flow and directionality were generally employed to corroborate other signs of dysfunction, but not to diagnose TIPS dysfunction in isolation.
Shunt venography and transjugular intrahepatic portosystemic shunt revision
Patients who exhibited return of clinical symptoms and/or who had abnormal Doppler US exams were referred to IR by the hepatology service for shunt venography and possible revision. Shunt venography and revision were undertaken in the IR suite using intravenous moderate sedation. From a right internal jugular vein access, a 7- to 10-Fr sheath was placed. The TIPS was then traversed using an angled catheter (MPA; AngioDynamics, Latham, NY, USA) and guide wire (Glidewire; Terumo Interventional Systems, Somerset, NJ, USA) combination, followed by advancement of a 5-Fr multi-side hole catheter (PIG; Cook Medical) into the main portal vein. Simultaneous main portal vein and right atrial pressures were obtained. TIPS venography was then performed. TIPS venographic abnormalities included shunt stenosis or occlusion and/or elevation of the portosystemic pressure gradient, which was considered abnormal if >12 mm Hg.[5] Angiographic or physiologic evidence of shunt dysfunction prompted shunt revision, which consisted of TIPS angioplasty using a 10-mm balloon (Mustang Balloon Dilation Catheter; Boston Scientific, Natick, MA, USA) or shunt relining using a VIATORR® TIPS endoprosthesis (W.L. Gore and Associates) based on the discretion of the primary IR operator. After TIPS revision, patients returned to standard Doppler US surveillance as previously described.
Medical chart reviews and measured outcomes
Review of the electronic medical record and assessment of radiologic imaging studies–undertaken on a picture archiving and communication (PACS) system–were used to obtain data for this study. This review was undertaken by a trained medical student research associate alongside a Certificate of Added Qualification licensed IR with 8 years of attending physician experience.
The primary outcome measure of this study was the concordance of clinical symptoms, Doppler US examination findings, and TIPS venography results. For concordance rate calculation, clinical symptoms were dichotomously classified as present or absent, and Doppler US and TIPS venograms were categorized in a binary fashion as normal or abnormal. Concordance rates were calculated between paired clinical and imaging parameters. Classification performance was evaluated by sensitivity and specificity rates, which were calculated for clinical symptoms and Doppler US for ruling out or ruling in venographically abnormal shunts (used as the reference standard). Concordance rates and classification performance were assessed for the entire study cohort as well as for a study subset after exclusion of cases, in which the time between Doppler US examinations and corresponding shunt venography exceeded 14 days to reduce the potential for confounding of results secondary to the possible development of TIPS dysfunction during a protracted interval between examinations.
Secondary outcome measures of this study included TIPS hemodynamic success, defined as a final portosystemic gradient (PSG) measuring <12 mm Hg[13] after initial TIPS creation and 30-day procedure-related adverse events, which were classified according to the Society of Interventional Radiology Standards of Practice Committee classification of complications.[5]
Statistical analysis
Descriptive statistics was utilized to check for data normality and to characterize demographic features of the study cohort. Concordance rates and classification performance parameters were compared between the whole study cohort and a subset cohort using the Pearson's Chi-squared test. Statistical analysis was performed using a commercially available software package (SPSS version 18; SPSS Inc., Chicago, IL, USA).
RESULTS
Transjugular intrahepatic portosystemic shunt procedures
Stent-graft TIPS creation was hemodynamically successful in 39/41 (95%) cases, with a median final PSG of 8 (range 3–14) mm Hg. Variceal embolization was performed in 11/41 (27%) cases. Thirty-day procedure-related adverse events included hepatic encephalopathy (HE) in 21/41 (51%) and liver insufficiency in 3/41 (7%) cases. HE was largely minimal or mild, with 16/21 (76%) patients categorized as Grade 1 or 2, while 5/41 (24%) patients were classified as Grade 3 or 4. No patients required shunt reduction.
Clinical findings, Doppler ultrasound examinations, and transjugular intrahepatic portosystemic shunt venography
Forty of 52 (77%) patients were symptomatic, with 32/40 (80%) having recurrent or persistent ascites or hydrothorax, and 8/40 (20%) suffering from recurrent variceal bleeding. The 41 patients in the study cohort underwent a total of 159 Doppler US examinations (median 2, range 1–9 per patient). The median time between the primary TIPS creation and Doppler US examination preceding TIPS venography was 179 (range 2–1814) days. Of the 52 US examinations accompanying TIPS venography, 33 abnormalities (64%) occurred among 26 patients. Decreased peak shunt velocity was the most commonly cited abnormality (n = 16, 48.5%; median peak shunt velocity = 51.5 cm/s, range 10–74 cm/s), followed by abnormally high peak shunt velocities (n = 12, 36%; median peak shunt velocity = 260 cm/s, range 197–311 cm/s) and shunt occlusion (n = 5, 15%).
The median time between Doppler US examinations and corresponding shunt venography was 3 (range 0–62) days. Twenty of 52 (39%) TIPS venography studies were abnormal. Of these, 8 (40%) had hepatic venous end stenoses, 4 (20%) had shunt occlusion, 3 (15%) had PSG elevation, 3 (15%) had tumor (hepatocellular carcinoma) invasion into the TIPS, and 2 (10%) showed portal venous end stenoses. For those patients with angiographic abnormalities requiring revision, prerevision PSG measured 18 (range 6–42) mm Hg and postrevision PSG measured 8 (range 2–14) mm Hg. Revision procedures were hemodynamically successful in 35/36 (97%) cases.
Concordance rates and classification performance
A total of 52 paired clinical examinations, US studies, and TIPS venograms were assessed. The concordance rate between clinical symptoms and TIPS venography was 48% (25/52), while the agreement rate between Doppler US examination and shunt venography was 65% (34/52) [Figures 1–3]. Clinical symptoms and the US concurred in 60% (31/52) of the cases. Notably, in the current series, US surveillance changed medical management in only 1/52 (2%) cases. In this instance, surveillance US performed on an asymptomatic patient revealed evidence of TIPS occlusion, which was corroborated at venography.
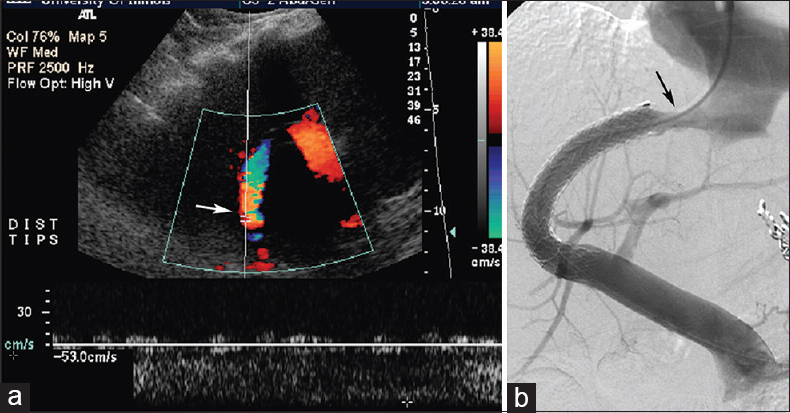
- Status of a 62-year-old woman post-transjugular intrahepatic portosystemic shunt for variceal hemorrhage with recurrent bleed and Doppler ultrasound abnormality 21-month postprocedure. Doppler ultrasound (a) reveals abnormally low shunt velocity measuring 53 cm/s (arrow), and concordant transjugular intrahepatic portosystemic shunt venogram (b) performed 3 days later shows hepatic venous end shunt stenosis (arrow).

- Status of a 56-year-old asymptomatic woman post-transjugular intrahepatic portosystemic shunt for hepatic hydrothorax and Doppler ultrasound abnormality 3-month postprocedure. Doppler ultrasound (a) demonstrates abnormally high shunt velocity measuring 204 cm/s (arrow), and discordant transjugular intrahepatic portosystemic shunt venogram (b) performed 3 days later shows patent transjugular intrahepatic portosystemic shunt with no abnormality (arrowheads), with portosystemic gradient measuring 12 mm Hg.
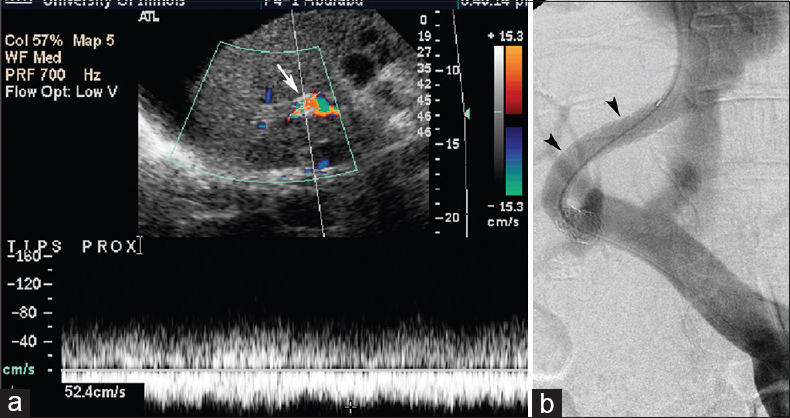
- Status of a 61-year-old man post-transjugular intrahepatic portosystemic shunt for ascites with recurrent ascites and Doppler ultrasound abnormality 3-month postprocedure. Doppler ultrasound (a) demonstrates abnormally low shunt velocity measuring 52 cm/s (arrow) (of note, Doppler angle depicted likely results in slight velocity overestimation), and discordant transjugular intrahepatic portosystemic shunt venogram (b) performed 2 days later shows patent transjugular intrahepatic portosystemic shunt with no abnormality (arrowheads), with portosystemic gradient measuring 11 mm Hg.
The sensitivity of clinical symptoms and Doppler US for the detection of venographically abnormal shunts was 80% (16/20) and 85% (17/20), respectively. Both clinical symptoms and the US had low specificity (25%, 8/32 and 50%, 16/32) for ruling in venographically abnormal shunts.
Subset analysis
Exclusion of cases, in which the time between Doppler US exams and corresponding shunt venography exceeded 14 days, yielded a cohort subset of 35 patients who underwent a total of 43 paired clinical examinations, US studies, and TIPS venograms. The median time between Doppler US examinations and corresponding shunt venography was 2 (range 0–13) days. Patients were symptomatic in 33/43 (77%) cases, 28/43 (65%) US exams were abnormal, and 14/43 (33%) TIPS venograms were abnormal. The concordance rate between clinical symptoms and TIPS venography was 42% (18/43), while the agreement rate between Doppler US examination and shunt venography was 67% (29/43). Clinical symptoms and the US concurred in 61% (26/43) of the cases. The sensitivity of clinical symptoms and Doppler US for the detection of venographically abnormal shunts was 79% (11/14) and 100% (14/14), respectively. Both clinical symptoms and US had a low specificity (24%, 7/29 and 52%, 15/29) for ruling in venographically abnormal shunts. Comparison of concordance rates and classification performance parameters between the whole study cohort and subset cohort yielded no statistically significant differences in rates (P > 0.05 for all comparisons), indicating similarity in outcomes between full and reduced patient samples.
DISCUSSION
Doppler US represents the primary method for noninvasive surveillance of TIPS patency in contemporary clinical practice.[5] However, current US protocols and sonographic criteria for the detection of TIPS malfunction are largely based on the outcomes from the bare-metal stent era. One of the initial studies of US for the detection of shunt dysfunction in bare-metal stents found 78% sensitivity and 99% specificity on the basis of mid-shunt velocity thresholds of <50 cm/s.[4] Various protocols and criteria studied for bare-metal stent dysfunction replicated these initial findings while also recommending the use of several US criteria to increase the accuracy of the examination.[14] Such parameters included interval changes in shunt velocities, measurement of portal vein velocity, and reversal of portal venous flow direction.[14]
While such experiences established a role for US surveillance of bare-metal stent TIPS, studies on US monitoring in the follow-up of TIPS created with stent-grafts have been less clear, with varying accuracy, predictive value, and clinical influence. In a 2005 prospective study involving 18 stent-graft TIPS and an accompanying 42 US examinations, Abraldes et al. published sensitivity and specificity rates of 100% and 59%, respectively, with US dysfunction criteria defined by mean maximal portal vein velocities <28 cm/s when flow was hepatofugal or <39 cm/s when flow was hepatopetal.[15] In 2006, Carr et al. retrospectively reported an US/venography concordance rate of 53% across 15 paired US and TIPS venography studies, and described that surveillance US detected a TIPS abnormality in only 1/88 (1%) asymptomatic patients undergoing routine follow-up using criteria including shunt velocity <60 cm/s or >200 cm/s, main portal vein velocity beneath 40 cm/s, a change in shunt velocity (decrease of more than 40 cm/s or increase of more than 60 cm/s), or new velocity gradient.[6] In a 2010 prospective investigation, Huang et al. communicated that only 7/30 (23%) patients with abnormal US examinations (defined by shunt velocity <50 cm/s or >250 cm/s or main portal vein velocity less than two-thirds baseline) showed TIPS venographic abnormality.[7] In 2013, Engstrom et al. reported sensitivity and specificity rates of 77% and 42%, respectively, across a retrospective cohort of 126 patients (83 with e-PTFE TIPS) using peak shunt velocity <90 cm/s, >200 cm/s, or absence of flow, as criteria for TIPS dysfunction.[8] Authors have also commented on the limited ability–1 of 88 (1%) cases in one instance[6] and 1 in 30 (3%) cases in another[7]–of US surveillance to change medical management of patients, especially symptomatic patients, with shunt dysfunction.
The current study aimed to assess the value of Doppler US in e-PTFE stent-graft TIPS surveillance by examining concordance between clinical symptoms, US examinations, and venography studies. The results indicated acceptable sensitivity rates for the US and clinical symptoms in detecting shunt dysfunction, but a poor specificity of either of these measures for TIPS dysfunction. Moreover, the current investigation uncovered low-to-moderate concordance rates between clinical symptoms or Doppler US and TIPS venography. In all, the findings—also corroborated in a subset cohort of cases in which Doppler US and TIPS venography were performed within 14 days of each other—suggest that clinical symptoms and US parameters have suboptimal predictive capacity for venographic findings. To this end, clinical employment in symptomatic individuals, namely those who demonstrate return of portal hypertensive complications, is probably not useful given that these patients will likely progress to TIPS venography regardless of the Doppler US findings. Application of an imperfect diagnostic study in the asymptomatic population is more challenging to consider: A normal US study in the setting of a stenotic shunt risks overlooking a meaningful abnormality that may spur a consequential clinical event (such as variceal hemorrhage) may have been averted by timely intervention, while an abnormal US study in the setting of a patent shunt may subject a patient to an unnecessary interventional procedure. Being a conservative IR practice, the current authors employ surveillance with the intent of potentially avoiding portal hypertensive events, but at the expense of performing more venography.
As a screening study, it is most important that surveillance Doppler US should have high sensitivity for the detection of shunt dysfunction to properly allocate patients—especially those who are asymptomatic so that intervention may be pursued prior to symptom development—to subsequent TIPS venography. While this may involve loosening velocity criteria to achieve ample detection capacity, other radiologic imaging modalities may also have a role in TIPS surveillance. To this end, magnetic resonance (MR) imaging—which has been reported for shunt[16] and portal venous system[17] evaluation in limited publications—offers a feasible alternative approach that has advantages of noninvasiveness, user independence, and lack of ionizing radiation. Future research into this approach may yield a viable alternative to the current Doppler US surveillance practices.
In addition to questions raised regarding the ability of US surveillance to affect clinical management, recent work has also revealed significant differences in flow mechanics in stent-grafts versus bare-metal stents. In 2013, Engstrom et al. described significantly higher flow velocities in normally functioning stent-graft TIPS compared with bare-metal TIPS (197.8 vs. 148.5 cm/s, P = 0.026), but no difference in velocities in malfunctioning stents.[8] Similarly, Huang et al. reported higher mid-shunt velocities in stent-graft TIPS compared to bare-metal stent TIPS (139 vs. 98 cm/s, P < 0.05).[7] Authors have also reported significantly higher, around 5–15 cm/s, velocities in main portal veins associated with stent-graft TIPS.[78] As a result, it has been suggested that the shunt velocity criteria developed for and tailored to the flow mechanics of bare-metal stents should be re-evaluated in considering the criteria for US surveillance of stent-grafts and numerical thresholds for the diagnosis of stent-graft TIPS dysfunction. Velocity criteria may be different for e-PTFE-covered VIATORR stent-grafts as compared to bare-metal stents secondary to inherent differences in baseline flow dynamics between devices, with stent-grafts distinguished by a smooth, e-PTFE-covered inner lining that limits luminal narrowing secondary to intimal hyperplasia, resistance to distortion by surrounding liver parenchyma or protrusion of liver tissue through stent interstices, and strong radial forces that allow for further gradual device self-expansion with accompanying decrease in flow resistance.[18]
Limitations
There were limitations to this investigation. First, the study contains retrospective, nonrandomized data from a single center with a relatively small patient sample size. Second, the patients in the study cohort had temporally heterogeneous follow-up in terms of Doppler US examinations and TIPS venography studies. Despite this diversity, all members of the patient cohort did have corresponding Doppler US and shunt venography studies generally performed in close temporal proximity. Third, variation in US technique (especially in patients with obese body habitus) and reporting may impact the study outcomes. Fourth, the study did not attempt to define or refine Doppler US criteria for stent-graft TIPS dysfunction. Future investigations may aim to determine the performance characteristics of various quantitative Doppler US thresholds in the accurate detection of stent-graft TIPS dysfunction. Fifth, the validity of the performance of classification parameters may be confounded given that the majority of patients in the current study were symptomatic and/or had an abnormal US examination.
CONCLUSION
Doppler US examination and clinical symptoms had low concordance rates with TIPS venography in this series, with acceptable sensitivity rates but with poor specificity rates. In all, the findings of this study suggest a need for improved noninvasive imaging methods for TIPS surveillance and suggest the need for optimization of US parameters tailored to stent-grafts or development of other noninvasive surveillance approaches, such as MR venography.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/29/186510
REFERENCES
- Long-term patency and clinical analysis of expanded polytetrafluoroethylene-covered transjugular intrahepatic portosystemic shunt stent grafts. J Vasc Interv Radiol 2015:261257-65.
- [Google Scholar]
- Clinical events after transjugular intrahepatic portosystemic shunt: Correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-303.
- [Google Scholar]
- Detection of transjugular intrahepatic portosystemic shunt dysfunction: Value of duplex Doppler sonography. AJR Am J Roentgenol. 1995;164:1119-24.
- [Google Scholar]
- Transjugular intrahepatic portosystemic shunts: Accuracy of Doppler US in determination of patency and detection of stenoses. Radiology. 1996;201:141-7.
- [Google Scholar]
- Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2016;27:1-7.
- [Google Scholar]
- Role of ultrasound surveillance of transjugular intrahepatic portosystemic shunts in the covered stent era. J Vasc Interv Radiol. 2006;17:1297-305.
- [Google Scholar]
- Comparison study of Doppler ultrasound surveillance of expanded polytetrafluoroethylene-covered stent versus bare stent in transjugular intrahepatic portosystemic shunt. J Clin Ultrasound. 2010;38:353-60.
- [Google Scholar]
- Covered transjugular intrahepatic portosystemic shunts: Accuracy of ultrasound in detecting shunt malfunction. AJR Am J Roentgenol. 2013;200:904-8.
- [Google Scholar]
- Transjugular intrahepatic portosystemic shunt using the FLUENCY expanded polytetrafluoroethylene-covered stent. Exp Ther Med. 2013;5:263-266.
- [Google Scholar]
- Transjugular intrahepatic portosystemic shunt for the treatment of medically refractory ascites. Diagn Interv Radiol. 2014;20:58-64.
- [Google Scholar]
- Root cause analysis of rebleeding events following transjugular intrahepatic portosystemic shunt creation for variceal hemorrhage. J Vasc Interv Radiol. 2015;26:1444-53.
- [Google Scholar]
- The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: Update 2009. Hepatology. 2010;51:306.
- [Google Scholar]
- Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168:467-72.
- [Google Scholar]
- Utility of color Doppler ultrasonography predicting tips dysfunction. Am J Gastroenterol. 2005;100:2696-701.
- [Google Scholar]
- Usefulness of 4D MRI flow imaging to control TIPS function. Am J Gastroenterol. 2012;107:327-8.
- [Google Scholar]
- MR-based visualization and quantification of three-dimensional flow characteristics in the portal venous system. J Magn Reson Imaging. 2010;32:466-75.
- [Google Scholar]
- Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239-48.
- [Google Scholar]






