Antenatal Umbilical Coiling Index and Newborn Outcomes: Cohort Study
Address for correspondence: Dr. Josephine Mwikali Ndolo, Vanderbilt University Medical Centre – Radiology, 1161 21st Avenue South, CCC-1121 Medical Center North, Nashville TN 37232-2675, USA. E-mail: josephine.m.ndolo@vanderbilt.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
We aimed to test the predictive value of antenatal umbilical coiling index (aUCI) among a prospectively recruited cohort of antenatal women.
Methods:
Women with singleton pregnancies were recruited at their second-trimester scan. Images of the umbilical cord were used to calculate the aUCI. Pregnancy and birth outcomes were recorded and statistical associations between aUCI and small for gestational age (SGA) using international standard birth weight centiles and preterm birth were investigated (n = 430).
Results:
aUCI results were consistent with the literature and showed good reproducibility between observers. Abnormal aUCI was not associated with SGA, but there was a statistical association with preterm birth (odds ratio 3.3 (95% confidence interval 1.4–7.7, P = 0.003). The sensitivity, specificity, and positive and negative predictive values for preterm birth were 47.6%, 76.9%, 9.6%, and 96.6%, respectively.
Conclusions:
The coiling index is unlikely to be useful in clinical practice as a screening tool for preterm birth owing to limited predictive value. We exclude a statistically or clinically significant association between abnormal coiling and SGA.
Keywords
Fetal biometry
fetal surveillance
intrauterine growth restriction
preterm birth
umbilical cord

Introduction
Coiling of the umbilical cord has been the subject of anatomical and sonographic study. In modern literature, Edmonds was the first to consider the anatomy and described an “index of twist.”[1] Based on a concept that abnormal vasculogenesis and/or development of the Wharton's jelly ground substance might be a marker for adverse fetal growth and obstetric outcomes, several studies of cords examined postnatally have explored possible associations between coiling and outcomes. As a basis for such studies, the normal range for coiling at birth following uncomplicated singleton pregnancies was established in a series of 122 births.[2] Extending the concept into the antenatal period, a detailed review considered the development and structure of the umbilical cord and examined the rationale and potential to take advantage of sonography to identify abnormal coiling in utero as a marker of fetal compromise. The review concluded that although promising, further evidence was needed to substantiate this approach.[3] The same authors subsequently reported a strong correlation between sonographic and postnatal measurement of umbilical coiling. There was an indication that the risk of small for gestational age (SGA) at birth and a need for intervention due to nonreassuring fetal status were higher in the presence of an abnormal coiling index, but the confidence intervals (CIs) around the risk estimates were very wide, leading the authors to conclude that larger studies would be needed to confirm useful predictive potential.[4]
Despite technical advances in imaging and other methods of fetal assessment, the available screening and diagnostic tools have important limitations leading to adverse outcomes as a result of failure to detect fetal compromise at the same time as a high rate of obstetric intervention for suspected compromise that turns out to have been unnecessary. Similarly, the available strategies for identifying women at risk of preterm birth have their limitations. We aimed to test the predictive value of antenatal umbilical coiling index (aUCI) among a prospectively recruited cohort of antenatal women.
Methods
Study site
Participants were recruited in the ultrasound unit at the Aga Khan University Hospital, Nairobi. This is a private not-for-profit teaching hospital serving a predominantly economically secure urban population. The clinical service includes both patients under the care of university faculty obstetricians and private practitioners. Further details of the hospital setting and population served have been reported in the context of another study undertaken in our institution.[5] Ethical approval was obtained from the Institutional Ethical Review Board.
Study population
Women of at least 18 years of age attending the hospital for an obstetric ultrasound examination who reported a gestational age between 18 and 24 weeks were invited to participate. The study inclusion criteria following the scan were a singleton viable fetus with biometry confirming a gestational age within the above range. The following were the criteria for exclusion from the study after the scan: gross fetal anomalies, oligohydramnios or polyhydramnios as defined by an amniotic fluid index of <5 cm or >25 cm, respectively,[6] technical difficulty with imaging the cord such as a suboptimal longitudinal image of the umbilical cord rendering an accurate aUCI measurement impossible, and presence of a single artery in the umbilical cord. Maternal medical conditions were not considered as exclusion criteria. A sample size calculation based on 80% power to detect a hypothesized SGA rate of 5% among participants with normal cord coiling versus 15% among those with abnormal coiling indicated a desired sample size of 436 participants.
Study procedures
Participants were recruited on attendance for ultrasound scans as described above. They were provided with an information sheet about the study and informed consent was obtained. Transabdominal ultrasound scanning was carried out using one of the five machines that were maintained according to the manufacturer's recommendations. The models used were GE Voluson P8 Logiq E9, Philips Epiq 7G, iU22, and HD 15. Fetal biometry was undertaken according to a standardized departmental protocol based on the INTERGROWTH-21st consortium procedures.[7] Six sonographers carried out study scans and were trained to assess the umbilical cord using a standard protocol, according to which images including a longitudinal view of the cord showing at least two complete segments (two arteries and one vein) were acquired excluding the two extremes of the cord (placental and fetal attachments). This middle portion of the cord has been shown to be the approximate arithmetic mean of the cord at the fetal and placental ends.[8] It should be noted that the practical inability to measure coiling over the entire length of the cord is an inherent limitation of the study. The images were captured and stored in the hospital's picture archiving and communication system and were available online for subsequent assessment of image quality, measurement, and calculation of the aUCI. After assessment of the acceptability of the images using the criteria described above, the distance between the coils was measured from the inner edge of an arterial or venous wall to the outer edge of the next coil along the ipsilateral side of the umbilical cord. The aUCI was calculated as a reciprocal of the distance between a pair of coils (aUCI = 1/distance in cm) as shown in Figure 1. MN performed all the calculations. If the umbilical coiling index (UCI) was too low to measure one complete coil in one view, the largest segment of cord without a complete coil was measured. The aUCI was then calculated as the reciprocal value of the mean of the two measurements of the pitch of one complete coil or as the reciprocal value of the largest length of umbilical cord without one complete coil [Figure 1].
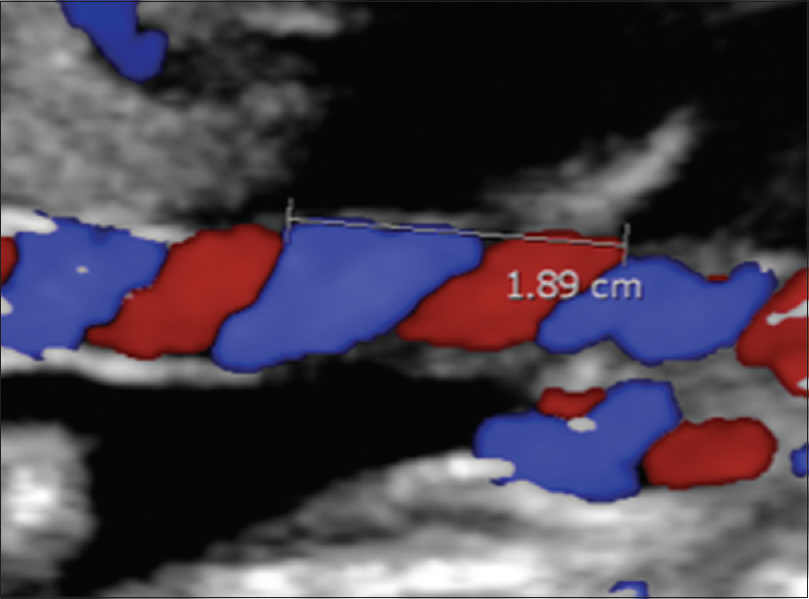
- Color Doppler ultrasound showing measurement of the umbilical coiling index from the inner edge of an artery to the outer edge of the same artery at the adjacent umbilical twist along the ipsilateral cord side. The umbilical coiling index was 0.52.
Only one image from each participant was included in the data set. Participants’ medical records were reviewed for maternal demographic characteristics, antepartum complications, and birth data. Variables retrieved include maternal age, gravidity, parity, gestational age at which the second-trimester ultrasound was performed, gestational age at delivery, mode of delivery, need for intervention at delivery, and neonatal birth weight.
Calculating interobserver variability
Interobserver variability was carried out between the principal investigator and a radiologist with experience in feto-maternal sonography. Every fifth umbilical cord assessed ultrasonographically was included to estimate the degree of agreement.
In addition, the AC1 statistic was also calculated as a further estimate of reliability.[9]
Outcome measures
Gestational age at delivery, birth weight, mode of delivery, and any history of complications were obtained from hospital records. For the classification of newborns as SGA, we used gestational age-specific centiles from the INTERGROWTH-21st study.[1011]
Data management and analysis
Data were entered and managed in the Microsoft Excel 2010 spreadsheet. Stata® version 11.2 (StataCorp., Texas, USA) was used for statistical analysis. Variables were tabulated and summarized into means or medians for continuous variables and percentages for categorical variables.
The baseline characteristics of maternal age and parity were compared between groups with normal and abnormal cord coiling to ascertain their comparability. The prevalence of SGA and preterm birth was calculated and presented as a percentage for each group with the relative risk and associated 95% CI. The sensitivity, specificity, and predictive value of aUCI for SGA and preterm birth were estimated as a guide to the potential clinical usefulness of the index. Furthermore, the risk differences between the two groups were adjusted for potential differences in their baseline characteristics. Chi-square test was used to determine the significance of differences between categorical variables and t-test was used to compare means, all using a 5% significance level.
Approval to undertake the study was obtained from the Aga Khan University Faculty of Health Sciences Research Ethics Committee. All participants signed a record of informed consent.
Results
Data were collected prospectively between December 2013 and October 2014 where a total of 602 pregnant women who fulfilled the inclusion criteria gave informed consent. Complete data were obtained from 442 women who either delivered at the Aga Khan University Hospital or for whom complete delivery records were available from other facilities. One late miscarriage and two intrauterine fetal deaths occurred. Data from 12 participants were excluded due to poorly obtained coiling indices whose longitudinal ultrasound images were deemed inadequate. 430 participants were included in the final analysis [Figure 2].
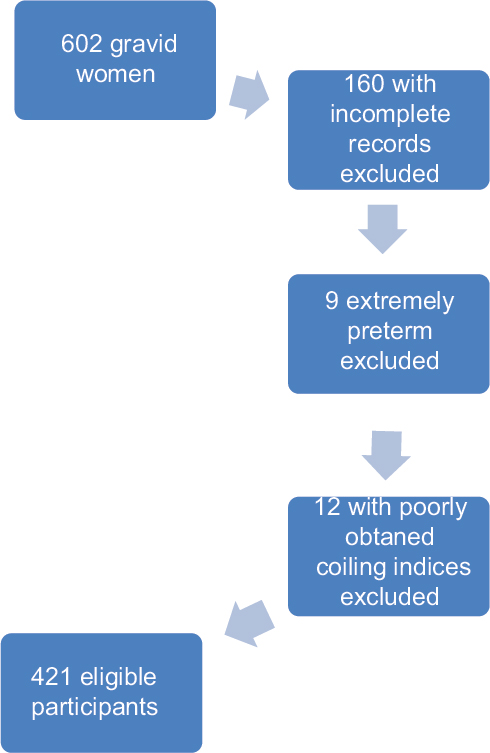
- A flow chart demonstrating the patient inclusion process.
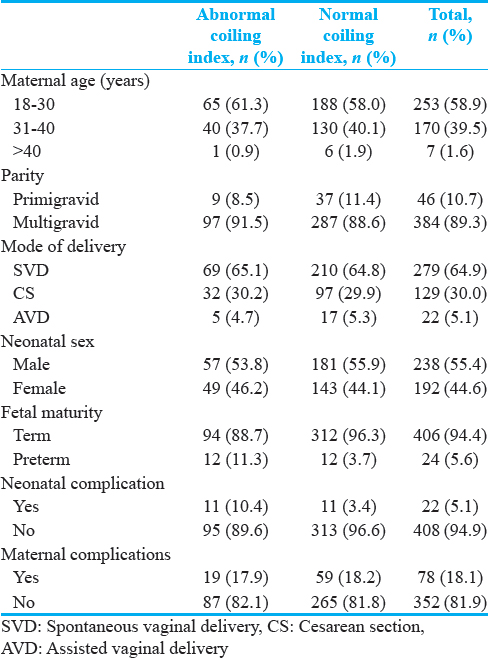
The range of the aUCI was from uncoiled (0) to 1.49, with a median aUCI of 0.43. Normocoiling in this study was defined by an aUCI between 0.21 and 0.59.[12] Of the 430 coiling indices that were calculated, 324 (75%) were normocoiled, 45 (11%) were hypocoiled, and 61 (14%) were hypercoiled. Table 1 shows the participant characteristics and clinical outcomes grouped by aUCI category.
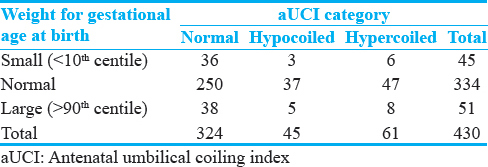
Using the intergrowth newborn weight for gestational age standards, 45 (10.5%) of the neonates were classified as SGA, 334 (77.7%) as normal for gestational age (NGA), and 51 (11.9%) as large for gestational age (LGA). The distribution of the SGA, NGA, and LGA in the normocoiled, hypocoiled, and hypercoiled groups is shown in Table 2. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of abnormal coiling for predicting SGA were 20%, 75%, 9.8%, and 87.4%, respectively. The odds ratio (OR) for the association between abnormal coiling index versus SGA at birth was 0.7 (95% CI: 0.3–1.6, P = 0.445). After adjusting for potential confounders using multivariable logistic regression models, there was no significant change in the above estimate with an OR of 0.6 (95% CI: 0.32–1.4, P = 0.26). The distribution of potential confounders and univariate probabilities and their distribution in the normal versus abnormal coiling are outlined in Table 3.
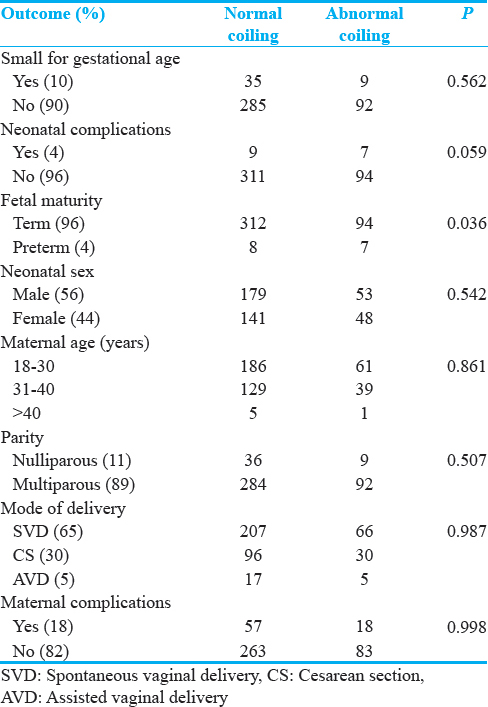
With regard to aUCI for the prediction of preterm birth, the sensitivity, specificity, PPV, and NPV were 47.6%, 76.9%, 9.6%, and 96.6%, respectively. The corresponding OR was 3.3 (95% CI: 1.4–7.7, P = 0.003).
The degree of agreement between the two observers independently computing the coiling index using 82 observations selected as every fifth umbilical cord image included was high, with a Kappa statistic of 0.95 and an AC1 statistic of 0.91.
Discussion
We have demonstrated a lack of association between abnormal antenatal umbilical coiling and fetal growth as reflected in SGA status at birth, but our findings indicate that there is a statistical association between abnormal coiling seen at the second-trimester scan and subsequent preterm birth.
Our results with regard to the range of observed indices of coiling are consistent with those noted in previous studies. Normocoiling was between 0.22 and 0.67 with a median of 0.43, which is comparable to previous studies where the cutoff values for hypo- and hypercoiled were between 0.2 and 0.6,[13] with a mean aUCI ranging from 0.3 to 0.42.[13141516] We observed a number of uncoiled umbilical cords (aUCI = 0). These were often associated with normal outcomes; similar findings were noted in previous studies.[17] A plausible explanation that has been put forward to explain this is that the second-trimester aUCI is a poor predictor of the degree of coiling in the third trimester or at birth, with an increased degree of coiling at delivery. This was demonstrated in an earlier study which found a poor level of agreement (kappa = 0.005) between the second trimester UCI and the values at birth with ultrasound having a poor sensitivity in detecting hypocoiling (17.3%) and hypercoiling (9.1%).[8] Naturally, this may affect the usefulness of aUCI as a predictive test in clinical practice.
In common with other reports with smaller participant numbers,[18] we found no association between SGA at birth and aUCI while some smaller studies have indicated a potentially useful association.[19] We undertook the study intending to achieve a number sufficient to confirm or refute such an association: our findings constitute “evidence of no association,” and we consider it unlikely that a further increase in the sample size would generate findings strong enough to point toward inclusion of this test to identify fetuses at risk of growth restriction. We did not undertake detailed clinical characterization of our participants at the time of scanning for aUCI to identify very high-risk clinical subgroups such as those with hypertensive disease or other medical conditions: our findings and interpretation are therefore relevant to a general unselected obstetric population.
It should be noted that the large study of Sharma et al.,[14] with 600 participants, did find statistical associations between abnormal coiling and low birth weight (rather than gestation-specific weight centiles as used in the present study). Their findings are likely to indicate an association with preterm birth rather than growth restriction. Taking data from their table of term and preterm births delivered vaginally, the PPV and NPV values would appear to be 52.8% and 97.6%, respectively. Our PPV of 9.6% was much lower, and at least in our African population, aUCI determination would not have clinical utility as a part of preterm birth risk screening. However, further exploration of this examination in combination with established approaches such as cervical length measurement might be worthwhile.
Almost perfect interobserver variability was observed in the present study with a Kappa statistic of 95.1% and an AC1 statistic of 0.91. This, like in other studies,[8] demonstrates that the measurement of aUCI is highly reproducible. However, while it has been suggested that abnormal umbilical coiling (hyper- or hypo-coiling) can be recognized during the fetal anatomic ultrasound survey in the second trimester without significantly increasing examination time,[13] our experience was that imaging for aUCI was not straightforward, especially in earlier gestations. Although we did not quantify the additional imaging time, the acquisition of at least two segments of umbilical cord coiling, with two arteries and a vein, away from the placental and fetal insertion so as to achieve the degree of reliability required was challenging and time-consuming. This can be explained by the significant variation that exists in the relationship of the umbilical vessels, especially depending on the ultrasound plane,[8] and is thus not simply a matter of sonographer training and experience.
Conclusion
Previous studies have reported contrasting findings with regard to potential associations between antenatal umbilical coiling and relevant perinatal outcomes, especially SGA and preterm birth. Our investigation had sufficient power to identify clinically important differences; while some potentially useful and statistically significant association is noted with regard to preterm birth, the practical limitations and low PPV do not indicate that priority should be given to further development of this approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/21/206697
References
- The spiral twist of the normal umbilical cord in twins and in singletons. Am J Obstet Gynecol. 1954;67:102-20.
- [Google Scholar]
- The umbilical coiling index in normal pregnancy. J Matern Fetal Neonatal Med. 2002;11:280-3.
- [Google Scholar]
- The umbilical coiling index, a review of the literature. J Matern Fetal Neonatal Med. 2005;17:93-100.
- [Google Scholar]
- Prenatal ultrasonographic prediction of the umbilical coiling index at birth and adverse pregnancy outcome. Ultrasound Obstet Gynecol. 2006;28:704-9.
- [Google Scholar]
- Implementation of the INTERGROWTH-21st project in Kenya. BJOG. 2013;120(Suppl 2):105-10. v
- [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 101: Ultrasonography in pregnancy. Obstet Gynecol. 2009;113(2 Pt 1):451-61.
- [Google Scholar]
- Intergrowth 21st Consortium. Ultrasound Operations Manual 2009
- Second-trimester ultrasonographic assessment of the umbilical coiling index. Ultrasound Obstet Gynecol. 2002;20:458-63.
- [Google Scholar]
- Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt 1):29-48.
- [Google Scholar]
- International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014;384:857-68.
- [Google Scholar]
- INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387:844-5.
- [Google Scholar]
- Assessment of umbilical cord coiling during the routine fetal sonographic anatomic survey in the second trimester. J Ultrasound Med. 2005;24:185-91.
- [Google Scholar]
- Ultrasound evaluation of abnormal umbilical cord coiling in second trimester of gestation in association with adverse pregnancy outcome. Am J Obstet Gynecol. 2005;193:387-94.
- [Google Scholar]
- Association of umbilical coiling index by colour Doppler ultrasonography at 18-22 weeks of gestation and perinatal outcome. J Obstet Gynaecol India. 2012;62:650-4.
- [Google Scholar]
- Early second-trimester low umbilical coiling index predicts small-for-gestational-age fetuses. J Ultrasound Med. 2001;20:1183-8.
- [Google Scholar]
- Sonographic evaluation of umbilical cord insertion with umbilical coiling index. J Clin Ultrasound. 1999;27:341-4.
- [Google Scholar]
- Evaluation of umbilical cord thickness, cross-sectional area, and coiling index as predictors of pregnancy outcome. Indian J Radiol Imaging. 2011;21:195-8.
- [Google Scholar]
- Sonographic estimation of umbilical coiling index and correlation with doppler flow characteristics. Obstet Gynecol. 1995;86:990-3.
- [Google Scholar]






